In experimental researches reduction of the dispersion coefficient of materials when pressure of working gas exceeds 1 Pa is observed. That is connected to increasing of probability of returning of the sprayed atoms at a target as a result of processes of outdiffusion and reflection.
The outdiffusion is diffusion returning to the target of the sprayed atoms having average kinetic energy Ер, equal to the average kinetic energy of atoms of inert gas (Еg). Thus it is obvious, that returning of the sprayed atoms to the target due to outdiffusion can occur from the distances considerably exceeding average length of their free path (λР). It is necessary to understand returning of the sprayed atoms to the target as outdispersion as a result of their dispersion on atoms of inert gas. This process occurs on distances from the target, not exceeding λР, and is characterized by distinction in kinetic energies of collide particles.
How much potent is the value of outdiffusion on the value of the dispersion coefficient, shows figure 5.13 and table 5.4.
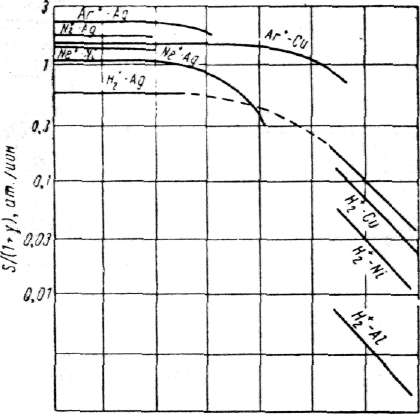 |
Fig. 5.13 Influence of outdiffusion of atoms on the dispersion coefficient at energy of ions 500 eV.
Table 5.4 Change of " the seeming dispersion coefficient “ with growth of productpod (atd = 1 sm), where ро =273р/Т - the pressure of gas resulted to Т=273 To;d - distance from the target to the surface of condensation
|
pod, torr·sm |
10-4 |
10-3 |
10-2 |
10-1 |
1 |
|
Кк/К |
0,99 |
0,96 |
0,55 |
0,10 |
0,01 |
At pod=10-2 torr·sm 45% of emitted atoms will come back to the target, and at 10-1 torr·sm - 90 % of incused atoms do not achieve a collector, which is away from the sample on distance only 1 sm.
With increasing of pressure of inert gas at dispersion of materials with weight of atoms mа, greater than weight of atoms of gas mg, the basic process of returning of sprayed particles on a target is outdiffusion as a result of which the effective speed of dispersion of a target and consequently, the speed of sedimentation of a film on a substrate is reduced. Therefore in practice for a choice of an optimum technological mode is very important to be able to estimate pressure of gas at which process of outdiffusion will begin. For calculations of dependence of the dispersion coefficient on pressure of gas and distances up to the surface of condensation in some cases use Penning and Maubsa's formulas which have assumed, that for the sprayed atoms it is possible to use the expression received by Gott and Poze for the description of diffusion in gas of evaporating atoms between two infinite planes [46]. The formula provides equality of average kinetic energies of sprayed particles Ер and atoms of gas Еg:
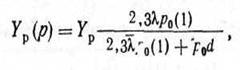 (5.3)
(5.3)
|
|
Fig. 5.14 Dependence of Yр from p0d for copper (а) and for nickel (b):
1 - experiment; 2 - calculation by formula (5.3); calculation by formula (5.7)
Where Yр - true dispersion coefficient of the material without taking into account influence of outdiffusion; λр0{1} - average length of free path of the sprayed atoms at pressure р=1 Pa and temperature 273 To; ро =273р/Т - the pressure of gas resulted to Т=273 To;d - distance from the target up to the surface of condensation. (5.3) dependences Yр of copper designed on the formula and nickel from product pod in comparison with experimental data give more than on the order the underestimated values pod for the beginning to return diffusion (fig. 5.14) [6]. Therefore the formula (5.3) cannot be used for engineering calculations at a choice of a technological mode of dispersion and geometry SS that shows necessity of development of new physical model of process of carry of the sprayed particles for the environment of working gas.
Average length of directed path Lк of the sprayed atoms on which they as a result of collisions with atoms of gas lose superfluous in comparison with Еg energy, it is possible to define under the formula
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.