Unfortunately, O2– is a moderately powerful reductant, leading to the decomposition of the organic cations in imidazolium- and phosphonium-based RTILs.305,306 Therefore, if these RTILs are used for electrochemistry, it is imperative to avoid contamination by oxygen. If both protons (water) and O2 are present, the results can be even more complicated. Numerous investigations of the reduction of O2 have been undertaken in RTILs. The data resulting from these investigations are summarized in Table 4. As found in most aprotic solvents, the O2/O2– electrode reaction is quasireversible. However, the heterogeneous kinetic data and diffusion coefficients resulting from the many prior investigations do show some differences. The reason for these variations is not clear, but it is probable that the concentration of dissolved O2 has not been established accurately and/or that the electrogenerated O2– reacts with impurities such as protons313 or water.305,309 It may also attack the organic cation.305,306 These differences could also result from

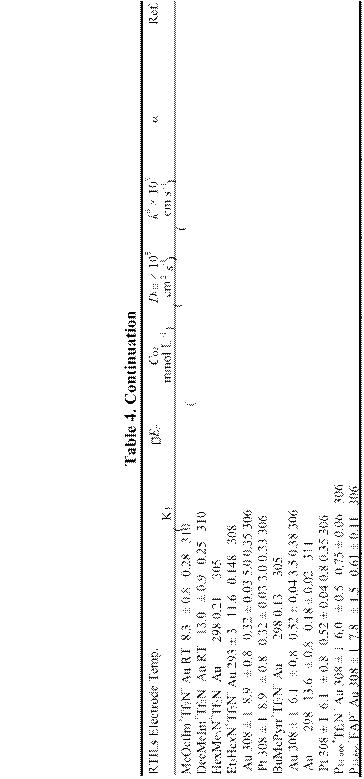
inconsistent efforts to account for the uncompensated resistance during these measurements. Overall, the presence of dissolved O2 can complicate electrochemical experiments in RTILs. Fortunately dissolved O2 can be easily removed under vacuum or by sparging with dry N2.
More than a decade ago, the equipment needed to carry out highfrequency AC impedance measurements was forbiddingly expensive. Therefore only a few electrochemists were well versed in the theory and experimental practice of AC impedance methods. However, the prospects for carrying out such studies have increased enormously because software-controlled equipment is now readily available at reasonable cost. AC impedance methods are widely used for measuring the conductivity of RTILs, but there is considerable variation among the data measured by different workers who have studied the same systems. Impurities aside, these deviations are likely caused by insufficient electrochemical knowledge. It is well-known that there are very strong interactions between anions and cations in most RTILs. Under such conditions, the Debye-Falkenhagen effect must be taken into account. Unfortunately, in many cases it has been ignored.
Conductivity cells are typically calibrated with aqueous KCl solutions prepared from highly purified water according to IUPAC recommendations.314 Depending on the cell geometry, the measured cell resistances may vary with frequency, ω, over the range from 100 Hz to 100 kHz. However, the cell resistance will usually show a linear dependence on the reciprocal of the square root of the frequency, ω-1/2. The frequency-independent resistances of the calibration solutions and the RTILs under investigation are obtained by extrapolating the cell resistance to infinite frequency.315 If such plots do not exhibit the requisite linear dependence, an empirical equation can be employed to obtain the frequencyindependent cell resistance.315 Normally the data resulting from such measurements exhibit high precision.
The self-diffusion coefficients of cations and anions in neat RTILs have been measured with pulsed-field gradient spin–echo NMR (PGSE–NMR).70,101,316-319 Such data can also be used to estimate transport numbers and ion mobilities, and it can be combined with other physicochemical properties to gain insight into the dynamics of ionic motion in RTILs.
A recent article by Earle et al.320 reported on the distillation and volatility of several common RTILs. It is generally held that most RTILs do not exhibit significant vapor pressure at room temperature. In practice this is true, but contrary to popular belief, many ionic liquids can be distilled under high vacuum with little or no decomposition in the temperature range of 473-573 K. The rate of distillation is roughly proportional to the molecular weight of the RTIL.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.