Nano-ordered deposits of pure metals, such as Mg,399,400 Ti,401 and Si,426-428 have been produced in these RTILs systems. These reports are noteworthy because these metals cannot normally be produced in metal halide–organic halide RTILs. However, additional investigations will be required to achieve the practical application of this technology. Katase, et al.449 have investigated Cu-Sn formation using the interdiffusion reaction between a Cu substrate and Sn deposited in n-HexMe3N+Tf2N-. The rate-determining step of this process was the interdiffusion reaction, and the activation energy for this reaction was estimated to be 58 kJ mol-1.
As a general rule, these systems are low cost compared to RTILs because they are formulated from off-the-shelf components that require only modest purification. For example, urea–chorine chloride mixtures are molten at room-temperature and display good environmental suitability.251 Both urea and choline chloride are inexpensive and available in bulk quantities. Urea-based RTMs containing the appropriate metal salts have been used successfully to prepare metal and alloy coatings on a variety of substrates. Most metal salts display good solubilities in these melts. There do not seem to be many difficulties associated with the preparation of solutions containing the large amounts of dissolved metal ions needed to deposit thick coatings. Some metal oxides also appear to dissolve readily in urea-based RTMs.253 However, the RTMs such as urea–chorine chloride mixtures typically show high viscosity.251 Thus, from a practical standpoint, these types of solvents must necessarily be employed at elevated temperatures.
Generally speaking, the electrochemical windows of most RTMs are better than those of most aqueous solutions, but inferior to that of non-chloroaluminate RTILs. Therefore, it is difficult to deposit base metals such as the rare earths and alkali metals from these solvents. In spite of these generalizations, some workers have reported the deposition of magnetic and semiconductive materials such as Sm-Fe,250 Sm-Co,257 and Cu-In-Ga-Se438 from the urea-based RTMs. Unfortunately, in many cases, these electrodeposits do not show magnetic and semiconductor properties unless they are annealed. Because most RTMs are usually very hygroscopic, care should be taken to handle them only under dry conditions.
The electrochemistry related to fuel cells based on RTIL electrolytes has been investigated since the early 1990’s.460 More recently, anhydrous protic RTILs and RTMs, which consist of HTf2N (or
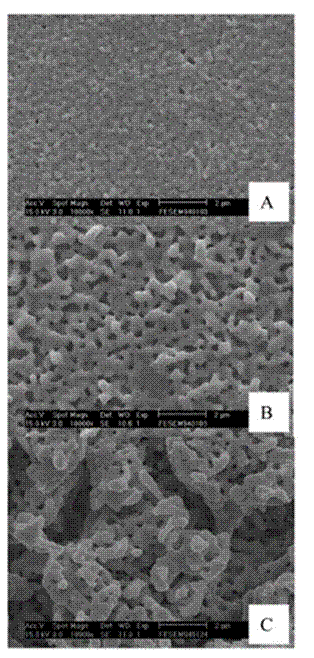
Figure 13. SEM images of Zn-Ag samples dealloyed after Zn electrodeposition on a Ag substrate. The sample areas covered by each micrograph are: (A) 0.64, (B) 5.12, and (C) 20.48 C cm-2. Reprinted with permission from Ref. 417. Copyright (2006) American Chemical Society.
HTf) and imidazole-based tertiary amines, have been proposed as solvents for PEM fuel cells.461 (Technically speaking, these solutions would only be a RTIL if the two components are mixed in equal proportions, otherwise they would simply be a RTM.) Most conventional PEM fuel cell systems suffer decreased performance at higher temperatures because of water evaporation. Non-aqueous proton conductive solvents have been proposed as an answer to this problem. There are many efforts to replace the conventional solvents used in PEM fuel cells with proton conductive RTILs (and RTMs). In addition, several research groups have focused on the advantages of RTILs in these applications and have proposed novel fuel cell systems based on the reactions that are inherent in RTILs such as imidazolium salts,460 AlCl3–EtMeImCl,462 and EtMeImF(HF)n.463,464 In this Section, we describe some of these electrode reactions and the operation of fuel cells based on RTILs.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.