the Fc+/Fc couple, bis(biphenyl)chromium couple (BCr+/BCr), or other metallocenes.324 The number of research groups using this approach seems to be increasing. But unfortunately, Fc+ may react with some RTILs38,325,326 and with traditional organic solvents (DMF and DMSO).327 If the decomposition of one of the reference compounds takes place during the electrochemical measurements, standard electrode reactions such as the aforementioned Ag+/Ag couple or the I–/I3– couple can be used as an alternative to Fc+/Fc. Figure 6 shows the electrochemical windows of n-HexMe3N+Tf2N– and conventional organic solvents calibrated relative to the Fc+/Fc standard electrode potential.328
As electrochemical solvents, most RTILs are not especially conductive. In fact, at room temperature, one of the very best salts, e.g., EtMeIm+F(HF)2.3-, exhibits a specific conductivity akin to that of a 1.0 mol L-1 aqueous KCl solution. Unfortunately, there are a
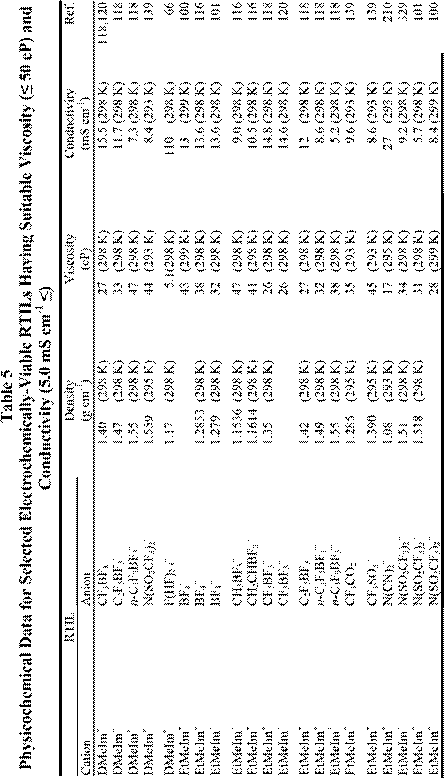

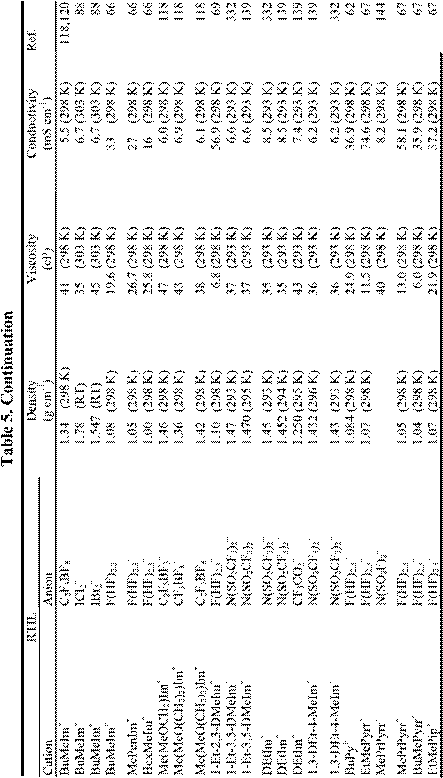
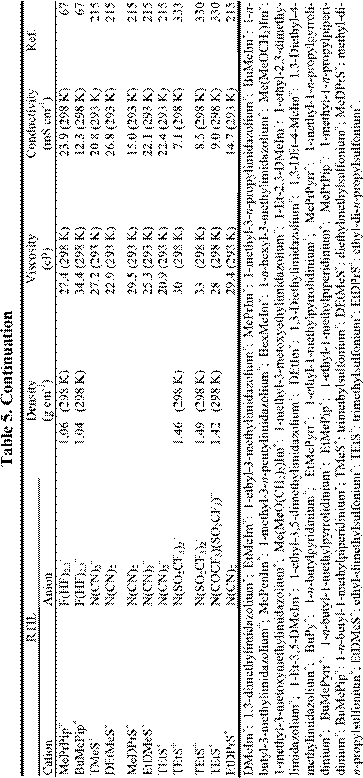
number of electrochemically favorable RTILs having useful conductivities (~ 5.0 mS cm-1) and low viscosities (~ 50 cP); some examples are given in Table 5. Even when employing the salts in this table, a substantial potential error can be expected during controlled potential measurements due to the effects of uncompensated resistance, Ru, between the WE and RE, despite the most careful electrochemical cell design. (This is rarely a concern at small macroelectrodes in conventional high-melting inorganic molten salts or ionic liquids, which tend to be very conductive.). As pointed out in a recent monograph,288 this effect is most noticeable in RTILs under conditions of high current density or large electrode area. In aqueous solutions, the potential error or iRu drop is often minimized by placing the RE in a compartment with a Luggin capillary. The tip of this capillary is placed close to the WE surface. In principle, there is no reason why such an approach cannot be employed in RTILs, but the high viscosity of these ionic solvents makes the use of Luggin capillaries difficult in practice. Although careful attention to the cell design can eliminate some of the Ru, most of the better quality commercial potentiostats/galvanostats designed for use at moderate currents with macroelectrodes have provisions for electronic resistance compensation. That is, they have circuitry that provides positive feedback to the control amplifier circuit (albeit at the expense of potentiostatic stability) that artificially removes the potential error. In almost every case examined by the authors, the use of electronic resistance compensation in RTILs is sufficient to reduce the potential error to negligible levels.
As described in Section III, because invisible impurities derived from air such as water and oxygen affect electrochemical reactions in RTILs, electrochemical experiments with these solvents, even those thought to be hydrophobic, should be conducted under a dry, inert gas atmosphere. Electrochemical reactions at a relatively positive potential, e.g., Al deposition, can be safely carried out under dry nitrogen. It is no secret that the N2/N3– electrode reaction has been observed in inorganic molten salts or ionic liquids. For example, in molten LiCl-KCl, the standard electrode potential for this redox couple, E0N2/N3− , is estimated to be 0.382 V vs. Li+/Li at 723 K.334 Thus, when alkali metal deposition/stripping reactions or other electrode processes are investigated at very negative potentials, it is wise to use dry argon as an inert gas since nitrogen may be reduced to nitride ion, N3–, in RTILs under these conditions.
2. An Overview of the Techniques Used for Electrochemical Analysis in Ionic Liquids
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.