Fuel cells employing proton conductive RTILs and RTMs are the most common systems that have been investigated because the prospective electrochemical reactions in these cells are basically the same as those found in conventional PEM fuel cells.467,470-476 In addition, these anhydrous systems can operate at temperatures of more than 373 K. Unfortunately, very little comprehensive cell performance data has been produced for comparison to conventional fuel cell systems. Polymer electrolyte membranes that consist of the same quantities of 2,3-dimethyl-1-octylimidazolium triflate, and polyvinylidenefluoride-co-hexafluoropropylene (PVdF-HFP), and HTf (0.5 M) have been examined in a single fuel cell having a 10 cm2 active area with 0.926 mg cm-2 of a Pt catalyst at 373 K.475 The maximum power density of this cell was ca. 1.0 mW cm-2 (Fig. 14). Susan et al.472 reported an unoptimized single cell prepared from a PVdF-(1,2,4-triazole/HTf2N = 5/5) membrane with a 1.6 mg cm-2 Pt catalyst that generated a maximum power of 0.32 mW cm-2 at 0.970 mA cm-2.
A non-proton transport type fuel cell system has also been proposed. This cell is based on RTILs derived from 1,3-dialkylimidazolium cations and fluorohydrogenate anions denoted by (HF)nF–.463,464 This fuel cell system is relatively simple as illustrated in Fig. 15. What is remarkable is that this fuel cell does not involve the direct transfer of H+. The reactions that take place in this fuel cell are shown below:
For n = 1.3
Anode: H2 + 6 (HF)F– → 4 (HF)2F– + 2e– (30)
Cathode: 4 (HF)2F– + ½ O2 + 2 e– → H2O + 6 (HF)F– (31)
For n = 2.3
Anode: H2 + 8 (HF)2F– → 6 (HF)3F– + 2e– (32)
Cathode: 6 (HF)3F– + ½ O2 + 2e– → H2O + 8 (HF)2F– (33)
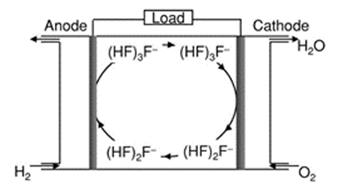
Figure 15. Operating principles of a fuel cell based on fluorohydrogenate ion conduction in EtMeIm+F(HF)2.3-. Reproduced from Ref. 464 by permission of ECS⎯The Electrochemical Society.
The overall reaction for n = 1.3 or 2.3 is, thus,
H2 + ½ O2 → H2O (34)
Figure 16 shows the open circuit voltage (OCV) of the fuel cell system illustrated in Fig. 15. The OCV was stable at ca. 1.1 V during experiments conducted over a period of 18 h and was almost independent of the n value.464 Surprisingly, under wet condition the cell performance was maintained without HF generation. A composite electrolyte consisting of poly-2-hydroxyethyl methacrylate and EtMeIm+(HF)2.3F–, which exhibits high conductivity, has great promise for use in the fabrication of a novel PEM fuel
cell system.477
Very recently, an innovative carbonate fuel cell system was proposed by a research group at the Georgia Institute of Technology, USA.478 This cell uses a carbonate anion exchange membrane as the electrolyte, and the cell can be operated with hydrogen or methanol as the anode gas. The proposed anodic reactions in this system are:
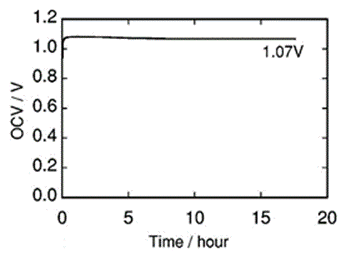
Figure 16. Open circuit voltage as a function of time for the fuel cell in Fig. 15. Reproduced from Ref. 464 by permission of ECS⎯The Electrochemical Society.
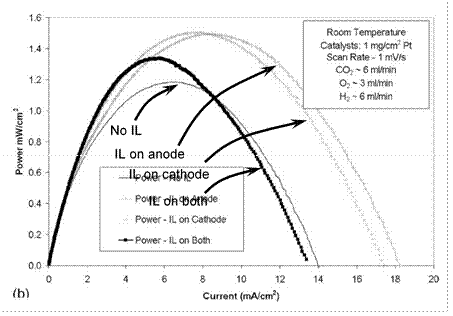
Figure 17. Power curve for room-temperature fuel cell modified with nBuMeIm+BF4- operating on hydrogen. Reproduced from Ref. 478 by permission of ECS⎯The Electrochemical Society.
for hydrogen
H2 + CO32– → H2O + CO2 + 2e– (35)
for methanol
CH3OH + 3 CO32– → 2 H2O + 4 CO2 + 6 e– (36)
The cathodic reaction is:
2 CO2 + O2 + 4 e-– → 2 CO32– (37)
Interestingly, applying n-BuMeIm+BF4- to this system improve the power density if hydrogen is used as the anode gas (Fig. 17). However, the reason why this RTIL enhances the cell performance is unclear, and there is no report that this system modified with the RTIL can be operated with methanol as the fuel. This room-temperature carbonate fuel cell is a notable energy conversion system with great promise.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.