High energy density batteries are widely used for electronics devices such as laptop computers and cell-phones. In particular, the Li-ion battery has been the focus of constant attention.24,479,480 The technology associated with Li-ion batteries is one of the most thoroughly studied topics in electrochemistry as a result of its significant economic impact. Unfortunately, the margin of safety for these particular batteries is less than that for many other battery systems. Because Li-ion batteries contain very reactive materials, they have a tendency for thermal runaway, leading to ignition and blowout. A safer nonflammable electrolyte that cannot contribute to thermal runaway is greatly desired. In addition, conventional secondary (rechargeable) cells based on Li metal do not reach their optimum performance level, owing to the instability of the electrolytes used in these cells toward Li metal. Not surprisingly, there is great interest in employing nonflammable and electrochemically stable RTILs and RTMs as electrolytes in these cells in an effort push the limits of current Li-ion battery technology.
Until several years ago, chloroaluminate RTILs were the major target for the Li battery electrolytes,24,25,481-484 but interest in the use of non-chloroaluminate RTILs has gradually increased in recent years. A large number of RTILs have been proposed for Li battery electrolytes, and actual Li rechargeable batteries have been described.144,148,156,261,330,397,485-496 Recent research involving Li and Li-ion rechargeable batteries based on RTIL electrolytes is described in Table 10. Those systems in which organic solvents were added to the RTILs are not included in this table. It is well known that adding organic solvents to RTILs often gives desirable results,391,397,499,503,504 but such mixed solvent systems are outside the scope of this article.
Although Li metal can be deposited from RTILs as indicated in Table 7, almost all RTILs react with Li metal to form a solid electrolyte interphase film (SEI) layer on the Li metal surface. The mechanisms by which these passivating layers are formed and the stability of these SEI layers vary with the types of RTIL, experimental temperature, and the presence or absence of organic solvents.330,391,499-503,505-507 Unfortunately, it seems that most RTILs are unstable during the cell discharge-charge process if the appropriate SEI layer does not form on the electrode. That is, the SEI
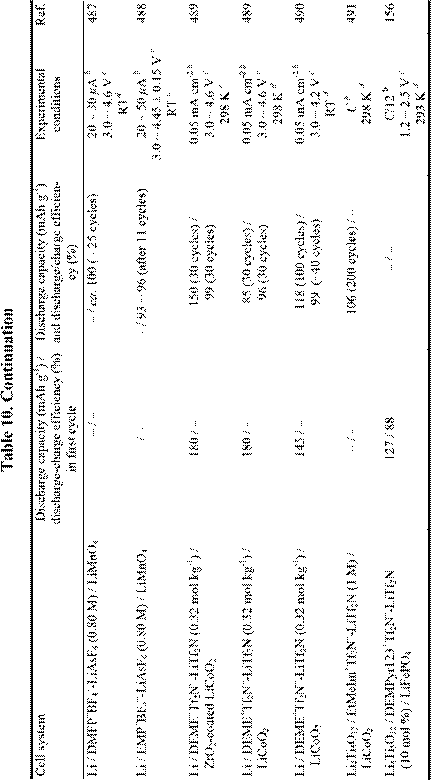
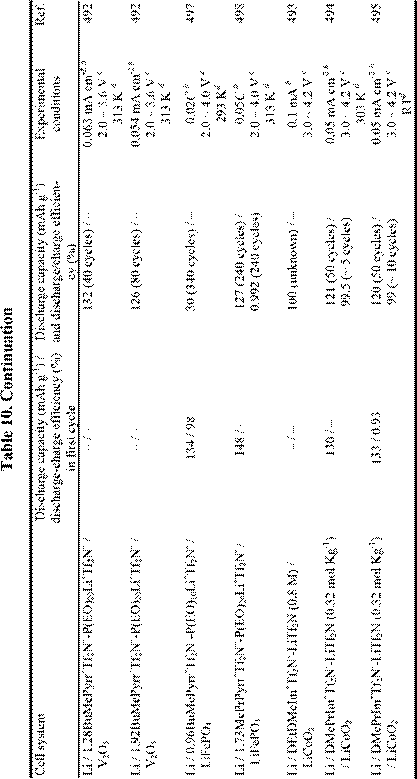
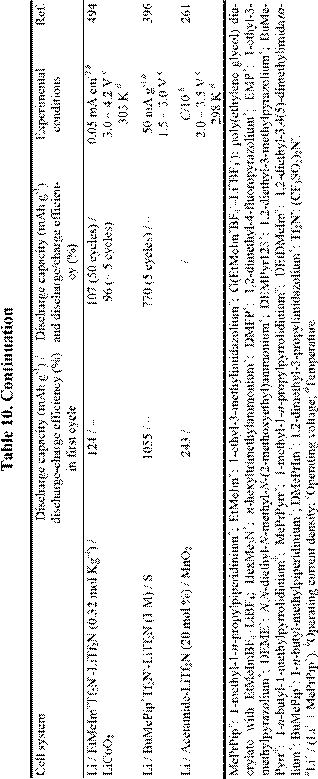
film that is produced on the anode during the charge/discharge process is inferior in stability to that obtained in conventional organic solvents. This incompatibility problem limits the cycling efficiency of such cells. Very recently, MacFarlane et al.506 have succeeded in elucidating the mechanism of film formation on Li negative electrode in RTILs based on N-alkyl-Nmethylpyrrolidinium ions and Tf2N-. On a negative note, Dahn et al.508 have concluded that RTILs are not necessarily safer solvents for Li-ion batteries.
Intercalation of the organic cation into a graphite electrode has also been observed.509 Thus, research and development involving RTIL-based Li batteries is no less difficult than that involving conventional solvents. As shown in Table 10, it is likely that the low capacity of the RTIL-based cells is caused by these undesirable solvent reactions. However, research with Li batteries based on ionic liquid electrolytes is in its infancy, and future success may be just around the corner.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.