III. FUNDAMENTAL PROPERTIES OF ROOMTEMPERATURE IONIC LIQUIDS
As a class of solvents, RTILs display many interesting and useful physical and chemical properties. However, newcomers to this field need to know that there are also limitations and special requirements associated with these ionic solvents that need to be understood before employing them as solvents for electrochemistry.
The use of RTILs at elevated temperatures requires information about their thermal stabilities. The thermal stability of a RTIL is usually defined through experiments carried out with differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), or differential thermal analysis (DTA). Although these methods are easy to use and show good reproducibility, the results obtained with these techniques, especially fast TGA scans (10 ~ 20 K min-1), do not always provide an accurate assessment of the thermal stabilities of the RTILs.35,269-274 Table 3 shows thermal decomposition rates at different temperatures for some popular RTILs compared to the decomposition onset temperature as measured by TGA-DTA. Obviously, significant decomposition occurs at temperatures considerably lower than the onset of the decomposition process. Therefore, electrochemical experiments should not be done at temperatures close to the decomposition temperature as determined by TGA/DTA, especially when the experiments are expected to last for a considerable period of time. Furthermore, several research groups have compared the thermal stabilities of selected RTILs in moist air and dry N2139,272,275 and found notable differences.272,275 These differences are directly related to the reactivity of the RTILs with atmospheric moisture. In addition to this factor, sample quantity,272 scan rate,272 and the material used to make the sample crucible271,272,276 can also affect the results.

Limited investigations have been carried out on the pyrolysis reactions associated with decomposition of the organic cations. The main products of the pyrolysis process seem to be related to the nucleophilicity of the anions and the basic skeleton of the cations. Two general decomposition models have been proposed for tetraalkylammonium cations: the well-known Hofmann elimination with the production of an alkene (Eq. 1) and the reverse Menschutkin reaction, leading to a tertiary amine and alkyl halide (Eq.
2):20,277
R4N+X– → R3NH+X– + CH2=CH2 (1)
R4N+X– → R3N + RX (2)
Similar investigations using phosphonium-based RTILs have been attempted, but the decomposition mechanisms are more complicated than for the tetraalkylammonium cations and often depend on the anion. The original literature should be consulted for more information.278,279
In the case of those RTILs based on dialkylimidazolium cations, the decomposition pathway most likely proceeds via SN1 (Eq. 3) or SN2 (Eq. 4) reactions:74,275
CH3 ⏐
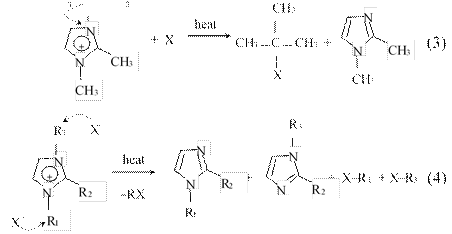 CH ⎯C⎯CH
CH ⎯C⎯CH
The reaction pathway depends on the nucleophilicity of the anions and the structure of the alkyl group on the nitrogen atom of
the imidazolium ring. Thus, if the anions are halides having strong nucleophilicity, and the cations have linear alkyl chains, the reaction will likely follow the SN2 pathway.74,275
Several groups have listed the thermal stabilities of the imidazolium cation-based RTILs. For example, Ngo et al.276 indicates PF6– > Beti– (bis(perfluoroethylsulfonyl)imide) > Tf2N– ≈ BF4– > Tf3C – ≈ AsF6– > I–, Br–, and Cl–. Awad, et al.275 gives the following order: PF6– > Tf2N– > BF4– > Br–, and Cl–, whereas Fredlake et al.280 proposes Tf2N– > Tf3C– > TfO– > BF4– > N(CN)2– > Br–. There is little doubt that the anion plays a crucial rule in the thermal stability. Overall, imidazolium-based cations are reported to be more stable than ammonium-based cations.276 The reason is unclear, but the aromatic properties of the imidazolium ring may contribute to the increased stability.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.