When one of the theoretical dimensionless current-time transients described above agrees well with the experimental data it is often possible to extract useful information about the deposition process such as the nucleation rate constant, the number density of active sites on the electrode surface, and the saturation density of nuclei by using the appropriate mathematical expressions.341-350
Those cases where the experimental current-time transients appear to fall between the theoretical transients for the limiting cases of progressive and instantaneous nucleation have also been considered,345,351-355 and a theoretical transient has been developed for this situation as well:356,357
⎛⎜ j ⎞⎟2 = tm { 1−exp[− xt /tm +α(1−exp−xt/αtm2 )]}2 (22)
![]()
⎜⎝ jm ⎟⎠ t { 1−exp[− x+α(1−exp−x/α)]}
In this equation, α and x are adjustable parameters that contain information about the number density of active sites and the potential dependent nucleation rate per active site. For the limiting cases of instantaneous and progressive nucleation, α approaches 0 with x ≈ 1.2564 and ∞ with x ≈ 2.3367, respectively. Figure 12 shows the dimensionless experimental data derived from the current-time transients for copper deposition on glassy carbon in a solution of Cu(I) in the 66.7 m/o urea–choline chloride room-temperature melt.354 Equation (22) was fit to the experimental data to give values of α and x of 0.422 and 2.140, respectively, with a correlation coefficient of 0.9922. In this case, α is a relatively large, suggesting that the deposition reaction is initiated mainly by progressive nucleation. A more comprehensive discussion of electrochemical crystallization can be found in Ref. 358.
(iv) Sampled Current Voltammetry
Several kinds of pulse voltammetric techniques have been proposed with the aim of avoiding charging current contributions to the overall current, thereby increasing the analytical detection limit of voltammetric measurements. These techniques, which are usually employed in conjunction with a dropping mercury electrode (DME), have been discussed at length in several prominent
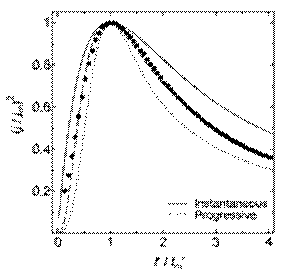
Figure 12. Examples of plots of t'/tm' vs. (j/jm)2 constructed from the current-time transients resulting from potential step experiments recorded at a GC electrode in a 66.7-33.3 mol % mixture of urea + choline chloride containing 20.1 mmol L-1 Cu(I). The theoretical transient was fitted to the experimental data by using the adjustable parameters, α and x, in Eq. (22).354
texts cited in Ref. 321. However, analytical techniques involving DMEs are seldom important in ionic liquid research, particularly if the RTIL is to be heated, but such pulse techniques can be advantageous at solid electrodes in RTILs. The most commonly applied pulse technique, sampled current voltammetry (SCV), is carried out by applying potential steps of increasingly negative (reduction) or positive (oxidation) amplitude to a stationary solid electrode to produce a series of potentiostatic current-time transients. The current for each transient is sampled at a designated time after the application of each step and then the potential is returned to the initial value potential while the solution is stirred. The advantage of the SCV technique is that the electrode diffusion layer and the electrode surface are renewed between each potential pulse. When the sampled currents are plotted as a function of the applied potential for a freely diffusing, i.e., kinetically uncomplicated system, the result is a current-potential curve identical to that obtained at a RDE that can be analyzed by using the current-potential expressions usually applied to polarographic and RDE waves. Because the electrode surface is renewed between data points, this technique can sometimes be used to produce well-defined currentpotential curves for systems that are intractable and do not produce useful voltammograms with conventional scanning methods at stationary and rotating electrodes. Several investigations in which this technique has been used to advantage in RTILs have been published by our research group.39,338,359
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.