2. Dialkylimidazolium Salts with Fluorohydrogenate Anions
Fluorohydrogenate RTILs, such as 1-ethyl-3-methylimidazolium fluorohydrogenate, EtMeIm+F(HF)2.3–, display low viscosity and high conductivity at room temperature. If properly prepared, these RTILs exhibit negligible release of HF gas and will not etch Pyrex® glassware.61-63 Because of these properties, they are excellent, if unappreciated, electrochemical solvents. Only modest skills with the safe handling of fluorine and/or HF are required to synthesize high purity EtMeIm+F(HF)2.3–. The starting material for this RTIL is EtMeImCl. Figure 4 shows a schematic of a vacuum line recommended for the synthesis of fluorohydrogenates. Because anhydrous HF reacts rapidly with borosilicate glassware, the vacuum line and reaction cell are made of fluorine-resistant materials such as SUS-316, FEP (fluoroethylene-propylene copolymer), PFA (perfluoroalkoxide polymer), or Teflon. Anhydrous HF (AHF) is obtained by contact with K2NiF6 overnight in a sealed cell. The volatile AHF is then transferred from the HF–K2NiF6 container to
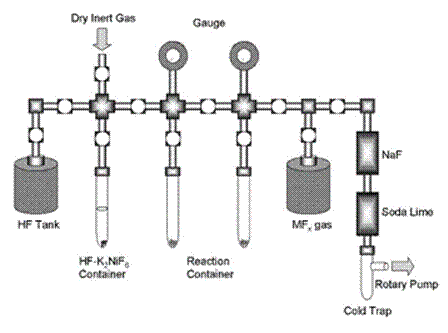
Figure 4. Diagram of a vacuum line used for the synthesis of fluorohydrogenate-based RTILs.
the FEP reaction tube containing EtMeImCl and a Teflon-coated stir bar by cooling the reaction tube with liquid N2. After melting the icy AHF in the reaction tube, the AHF (added in excess) reacts with the EtMeImCl and generates HCl gas. The resulting HCl can be eliminated by sparging the RTIL with dry nitrogen gas. This procedure should be repeated several times so as to ensure that no unreacted Cl- remains. Unreacted anhydrous HF is eliminated from the EtMeIm+F(HF)x– under vacuum by reducing the pressure to
7.5 × 10-3 torr (1 Pa) or less.
RTILs based on dialkylimidazolium salts with polyfluoroanions, such as MFx–, where M: B (x = 4); P, As, Nb, Ta (x = 6); W (x = 7), are normally prepared by using the corresponding aqueous acids or metal salts. This pathway leads to RTILs that are contaminated with water and/or metal chlorides. Such RTILs are difficult to purify. Recently, Matsumoto et al.131 have reported a novel procedure to make EtMeIm+MFx– salts by using EtMeIm+F(HF)2.3–.
The reaction between EtMeIm+F(HF)2.3– and the corresponding Lewis fluoroacid, MFx-1, is conducted in the reaction vessel shown in Fig. 4. There do not appear to be any significant side reactions, and the HF produced during the reaction can be readily eliminated from the resulting RTILs under vacuum. If EtMeIm+F(HF)2.3– is available, this is a quick and reliable method for preparing these salts.
4. Tetraalkylammonium Salts with Bis[(trifluoromethyl)- sulfonylimide Anions
Tetraalkylammonium salts with bis((trifluoromethyl)sulfonyl) imide (Tf2N-) anions, such as tri-n-butylmethylammonium bis ((trifluoromethyl)sulfonyl)imide, n-Bu3MeN+Tf2N–, are highly viscous RTILs.146 However, n-Bu3MeN+Tf2N– (386 cP at 303 K) in particular shows greater chemical and electrochemical stability than bis((trifluoromethyl)sulfonyl)imide salts based on dialkylimidazolium cations.150 Furthermore, it is very hydrophobic. This suggests that this RTIL might be a promising solvent for the chemistry of radioactive materials, radical chemistry, and high-energy density batteries.
The n-Bu3MeN+Tf2N- RTIL is prepared by mixing exactly equal molar amounts of n-Bu3MeNCl and LiTf2N in ultrapure water. (The former salt was purified by a procedure similar to that described above for EtMeImCl.) This solution is agitated at room temperature for 24 hours, and the resulting hydrophobic ionic liquid is extracted with ultrapure dichloromethane. This solution is washed with several portions of purified water until the wash water contains no chloride as determined by the addition of a drop or two of a dilute solution of silver nitrate. If necessary, the chloride content can also be evaluated with ion chromatography. Finally, the dichloromethane is removed by evacuating the solution at 1 × 10-3 torr for 24 h while it is heated to 373 K. The resulting RTIL is usually colorless and very dry (H2O < 3 ppm). All hydrophobic Tf2N--based RTILs can be prepared by using this same basic method.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.