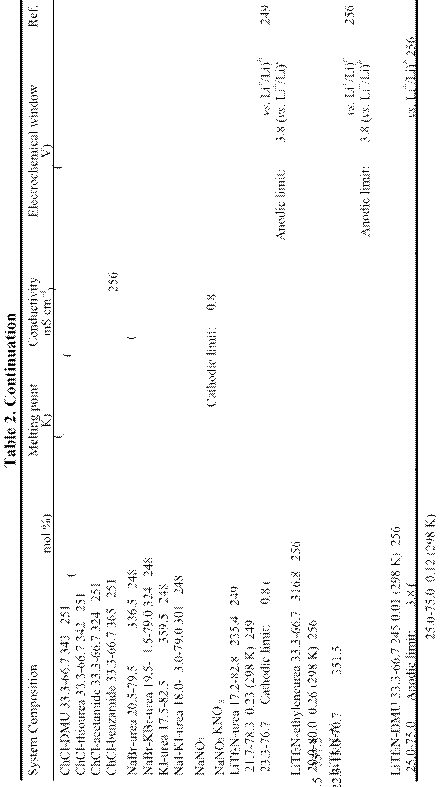
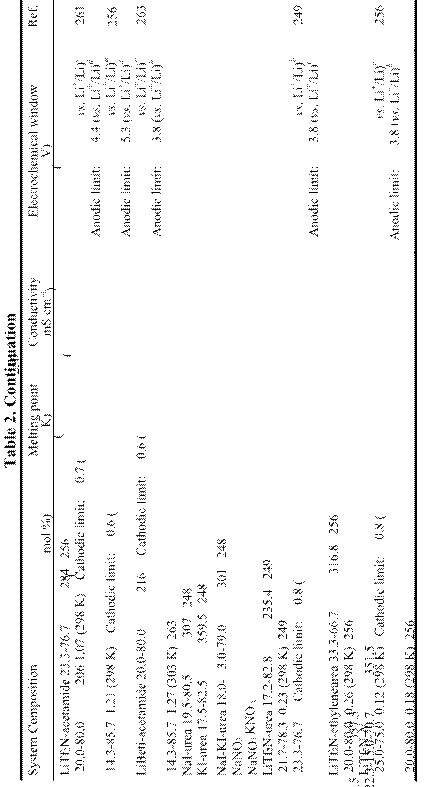
RT: m.p. ≤ 298 K; LT: 298 K < m.p. ≤ 373 K; Amm: Ammonium-based cation; Im: Imidazolium-based cation; P: Phosphonium-based cation; Pip: Piperidinium-based cation; Py: Pyridinium-based cation; Pyrr: Pyrrolidinium-based cation; S: Sulfonium-based cation.


II. SYNTHESIS AND PURIFICATION OF ROOMTEMPERATURE IONIC LIQUIDS
As noted above, there are many classes of non-chloroaluminate RTILs. However, not all of them are suitable for electrochemistry because they may be poorly conductive and highly viscous, or they may have a limited electrochemical windows. In this Section, we describe the general synthetic methods used to prepare and purify some selected RTILs, such as EtMeIm+F(HF)2.3–, EtMeIm+MFx– [M: B (x = 4); P, As, Sb, Nb, Ta (x = 6); W (x = 7).], and nBu3MeN +Tf2N-. These particular systems were chosen because they have been used as electrochemical solvents on many occasions. The various procedures that are used to prepare and purify other RTILs are far too numerous to list here, and they tend to be specific for the RTIL that is being prepared. The interested reader is referred to a recent article for more information.267
Among the various RTILs that have been used as solvents for electrochemistry, the greatest proportion is based on cations derived from 1,3-dialkylimidazolium chloride salts. The synthesis and purification of these materials were reported by Wilkes et al.75 more than two decades ago in one of the most highly-cited articles to appear in the journal Inorganic Chemistry. Although several newer procedures have been reported, the technique described in this seminal article is still a reliable and simple method to make highquality salts. Experience has shown that carefully prepared 1,3dialkylimidazolium salts are relatively easy to purify and ultimately lead to transparent RTILs with low levels of impurities. In fact, if nontransparent RTILs are obtained, they can be readily decolorized by passage of the RTILs dissolved in dichloromethane through a suitable chromatography column.268
A typical procedure for preparing one of the more popular salts, 1-ethyl-3-methylimidazolium chloride (EtMeImCl), is as follows: 1-ethyl chloride (600 mL) is condensed into a 1 L glass pressure vessel containing distilled 1-methylimidazole (300 mL) and dry acetonitrile (100 mL). The mixture is heated at 333 K with continuous stirring until the solution exhibits a milky-white color if the stirring is halted. The bulk of the unreacted ethyl chloride is
removed by sparging the reaction mixture with a stream of dry N2. The remaining traces of ethyl chloride are removed by evacuation. At this point, a large quantity of white crystals should be evident in the reaction vessel. These crystals are crude EtMeImCl. The crystals can be purified by precipitation from acetonitrile with ethyl acetate. Because this salt is very hygroscopic, a good quality vacuum line and an assortment of Kontes Airless-ware® flasks (Kjeldahl-shaped) and filter funnels with coarse porosity filters will greatly simplify this procedure. To carry out this purification step, the resulting crude EtMeImCl is first dissolved in warm (~333 K) acetonitrile. Ethyl acetate is then added to this solution. Upon cooling, fluffy white crystals of the salt precipitate from the solution. It may be necessary to scratch the inside of the flask with a glass rod or to shake the flask vigorously in order to induce precipitation. The salt is collected in an Airless-ware® filter funnel and dried under vacuum. Experience dictates that this procedure must be repeated at least three times in order to obtain a high-quality product. The final purification step involves melting the salt under vacuum (1 × 10-3 torr) at 373 K to remove the last traces of solvent.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.