A useful procedure for determining the electrochemical window of a RTIL is to record two cyclic voltammograms at a WE; one is initiated from the rest potential toward cathodic potentials, whereas the second is initiated from the rest potential toward anodic potentials or vice versa. Because sampling the potential limit of the RTIL is sure to produce some type of surface film, care should be taken to refresh or clean the electrode surface between scans. However, one must be mindful that the concept of an electrochemical window is somewhat subjective and depends on the level of the background current ascribed to the potential limit of the ionic liquid.
Most electrochemical texts provide little or no information about the desirable characteristics of the counter electrode (CE). When current is passed through an electrochemical cell, the function of the CE is to support the current that flows through the WE. The electrochemical reaction occurring at the CE must in no way affect the overall cell response. Therefore, the surface area of the CE should be considerably larger than that of the WE to ensure that all current limitations in the cell are due to the WE response. In some cases, it may be necessary to take into account the geometry and placement of the CE in order to provide uniform current distribution at the WE surface. Finally, some thought must be given to the electrode reaction that takes place at the CE. Will the products that are produced at the CE affect the cell reaction? In RTILs, the CE reaction is almost always the oxidative or reductive decomposition of the solvent. Thus, it is necessary to isolate the CE in a compartment that is separated from the bulk solution by a glass frit in order to limit the mixing of the contents of this compartment with the bulk solution. In addition, the volume of the CE compartment should be sufficient to contain enough of the RTIL to support the total charge passed during the experiment, or the CE
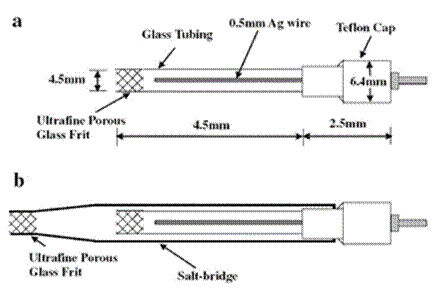
Figure 5. Diagrams of (a): a Ag+/Ag reference electrode for use with RTILs, and (b): this reference electrode inserted into a salt-bridge compartment. Reproduced from reference 323 with permission of Elsevier B.V.
reaction will limit the cell response. Typically, the CE is fashioned from a coil of Pt or W wire or a large surface area coupon or flag of the same materials. One of the authors has observed the successful use of a large stainless-steel spatula as the CE while visiting the laboratory of a prominent organic electrochemist!
At the present time, there is no universal reference electrode (RE) that can be used in all RTILs. However, Ag+/Ag couples pre- pared by immersing a clean Ag wire in a RTIL solution prepared from a soluble silver salt or by electrogenerating Ag+ show the most promise. Like the CE, the RE must be isolated from the bulk ionic liquid with a fritted glass barrier such as a glass frit or porous Vycor plug (Fig. 5a).323 If the leakage of Ag+ into the bulk solution is problematic, then it may be necessary to employ a salt bridge so that the hydraulic flow of the Ag+ reference electrode solution into the test solution is protected by two barriers. One such sample is depicted in Fig. 5(b).323 Another approach is to employ a simple quasireference electrode such as a Ag, Au, or Pt wire immersed in the RTIL and isolated from the bulk solution with a porous barrier. The potential of the quasi-reference electrode can be related to the one of the internal standards recommended by the IUPAC such as
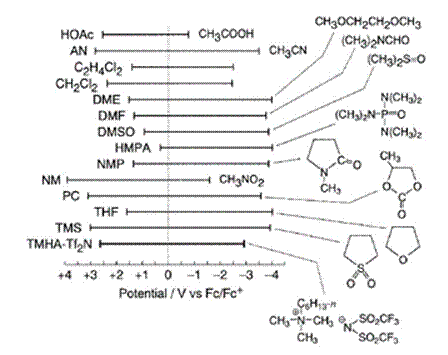
Figure 6. Comparison of electrochemical window of n-HexMe3N+Tf2N- (TMHATf2N) and some common organic solvents that have been used for electrochemistry.
All potentials are referenced to the standard electrode potential for the Fc+/Fc. Reproduced from Ref. 328 with permission of Kluwer Academic Publishers.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.