A great many room-temperature ionic liquids, abbreviated from this point forward as RTILs (plural form) or RTIL (singular form), have been prepared. These RTILs can be conveniently subdivided on the basis of the structure of the cationic component as depicted in Fig. 3, but it is considerably more difficult to classify them on the basis of anion structure due to the many different anions that have been used to formulate these materials (Table 1). The physicochemical properties of RTILs can be tuned across a broad spectrum by choice of the cations and anions. This is perhaps the most interesting and useful feature of this class of solvents. Those low-melting mixtures that fail conditions a. and b. listed above, but form room-temperature melts (RTMs), such as those based on zwitter ions,242-246 urea,8,247-257 or acetamide,255-266 also possess some unique physicochemical properties that make them suitable for electrochemistry as summarized in Table 2 and will also be discussed herein.
Although the popularity of RTILs (and some RTMs) cannot be disputed, there is often little understanding of the techniques and procedures that must be used to prepare and purify these materials. Therefore, we have compiled what we think to be the best information about these methods for each class of RTILs in the Section presented below. In other Sections of this article, we introduce fundamental electroanalytical chemistry in RTILs and discuss electrochemical technologies based on RTILs and RTMs, e.g., energy and materials science.
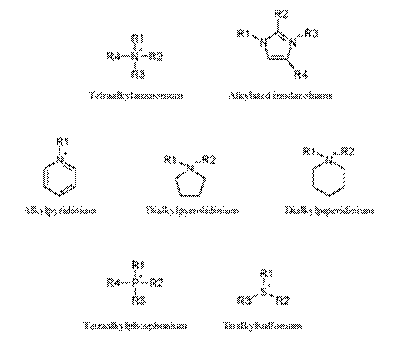
Figure 3. Common cations that have been used to prepare roomtemperature ionic liquids.
Typical Room-Temperature (RT) and Low-Temperature (LT) Ionic Liquids
|
Anions |
Cations |
Ref. |
|
F(HF)n– |
RT: Amm, Im, Pip, Py, Pyrr LT: Amm, Im |
61-72 |
|
Cl– |
RT: Amm, Im, P LT: Amm, Im, P |
73-80 |
|
ClO4– |
RT: Amm, Im LT: Amm |
74,81-83 |
|
Cl(HCl)n– |
RT: Im |
84-86 |
|
ClI2– |
RT: Im |
87 |
|
Cl2I– |
RT: Im |
88 |
|
Br– |
RT: Amm, Im, P LT: Amm, Im |
73,74,78,79, 81, 89 |
|
BrI2– |
RT: Im |
87,90 |
|
Br2I– |
RT: Im |
87,88,90 |
|
Br3– |
RT: Im, Py |
87,88,90 |
|
I– |
RT: Amm, Im, P, S LT: Amm, Im, S |
73,74,78,79, 82,83,91-96 |
|
Anions |
Cations |
Ref. |
|
I2n+1– |
RT: Im |
87,97 |
|
BH4– |
RT: Amm |
98 |
|
BF4– |
RT: Amm, Im, Py, Pyrr, Others LT: Amm, Im, Pip, Pyrr, Others |
73,77,82,83, 93,98-108 |
|
B(CN)4– |
RT: Im |
109 |
|
Borides (= BR1R2R3R4–) |
RT: Amm LT: Amm |
7,76,110-113 |
|
B(HSO4)4– |
RT: Im |
114 |
|
Tetraphenylborate (= BPh4-) |
LT: Amm |
81 |
|
Tetrakis[3,5-bis (trifluoromethyl)phenyl]borate (= B[Ph(CF3)2]4–) |
LT: Amm, Im, Py |
115 |
|
RBF3– |
RT: Im |
116 |
|
RfBF3– [Rf = CnF2n+1] |
RT: Amm, Im, Pip, Pyrr LT: Amm, Im, Pip, Pyrr |
103,107,117- 122 |
|
CH3CH(BF3)CH2CN– |
RT: Im |
123 |
|
PF6– |
RT: Im LT: Amm, Im, Pyrr, Others |
77,83,98,100, 124-128 |
|
(Rf)3PF3– [Rf = CnF2n+1] |
RT: Im, P |
129,130 |
|
AsF6– |
LT: Im |
100,125,131 |
|
SbF6– |
RT: Im |
131-134 |
|
TaF6– |
RT: Im |
135,136 |
|
NbF6– |
RT: Im |
135,136 |
|
WF7– |
RT: Im |
131 |
|
WOF5– |
RT: Im |
137 |
|
HCO2– |
RT: Amm |
98,138 |
|
HCO3– |
RT: Amm |
98 |
|
CH3CO2– (= AcO–) |
RT: Amm, Im, P |
80,98,99,139 |
|
CH3OCO2– |
RT(?): Im |
122 |
|
CH3CH(OH)CO2– (= Lac–) |
RT: Im LT: Others |
105,140 |
|
CH3CH=CHCO2– (= Crot–) |
RT: Amm |
98 |
|
CF3CO2– (= TA–) |
RT: Im, Others LT: Im, Others |
105,108,139 |
|
CH3SO3– (= MsO–) |
RT: Im LT: Im |
92,141 |
|
CF3SO3– (= TfO–) |
RT: Im LT: Im |
83,139,141, 142 |
|
C3F7CO2– (= HB–) |
RT: Im |
94,139 |
|
C4F9SO3– (= NfO–) |
RT: Im LT: Im, Pyrr |
139,143 |
|
(FSO2)2N– (= FSI) |
RT: Im, Pip, Py, |
144,145 |
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.