Electrochemical applications of RTILs include their use as solvents for the study of redox reactions and metal deposition and as electrolytes for a variety of technological applications, including surface finishing, electromechanical actuators, dye-sensitized photoelectrochemical cells, electrochromic devices, fuel cells, doublelayer capacitors, and nonaqueous batteries. Many of these applications were featured in a recent article.18 Electrochemical data for a variety of organic, organometallic, and inorganic solutes that have been studied in RTILs are given in Table 6. By the far, the most extensively studied solute in RTILs is oxygen, and so we have placed this data in a separate table (Table 4). From these tables, it
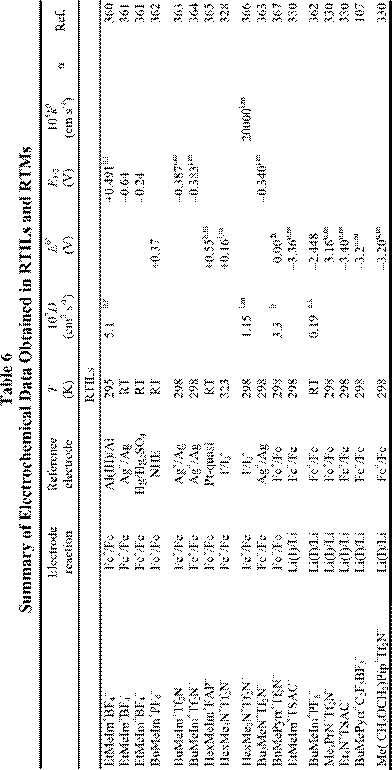
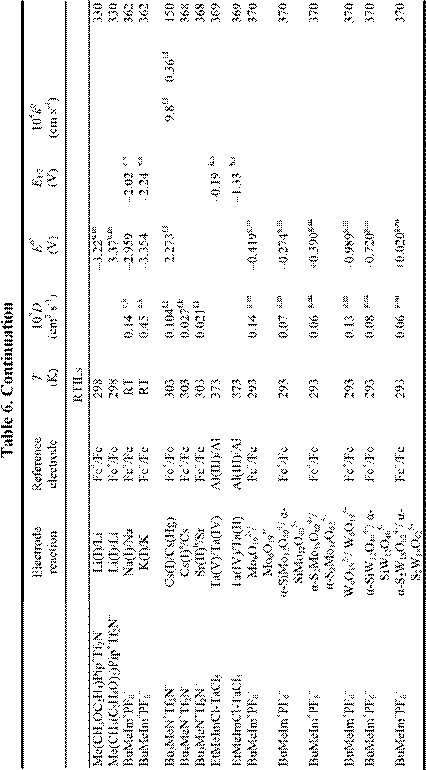
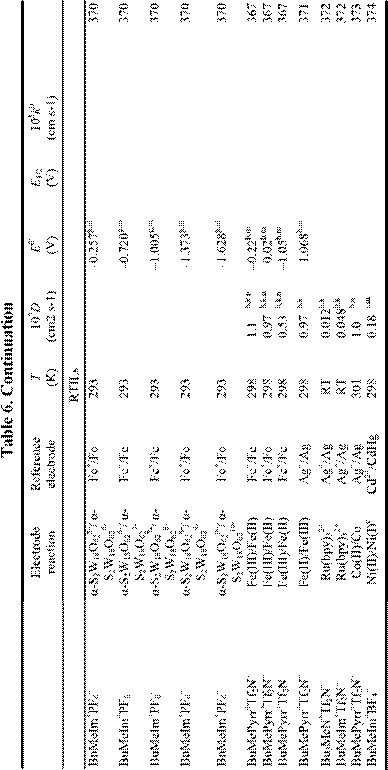
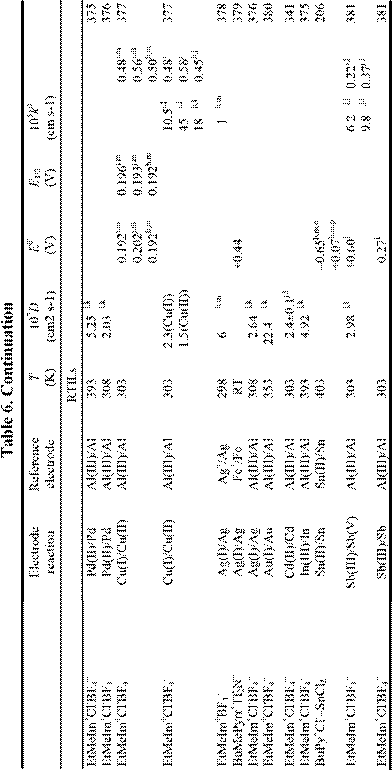
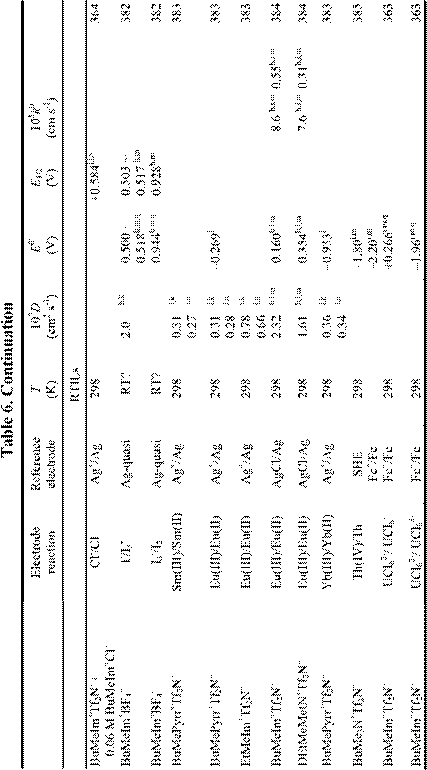
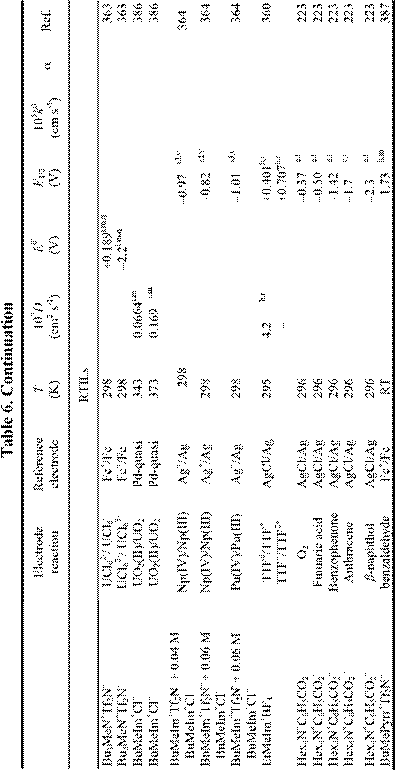
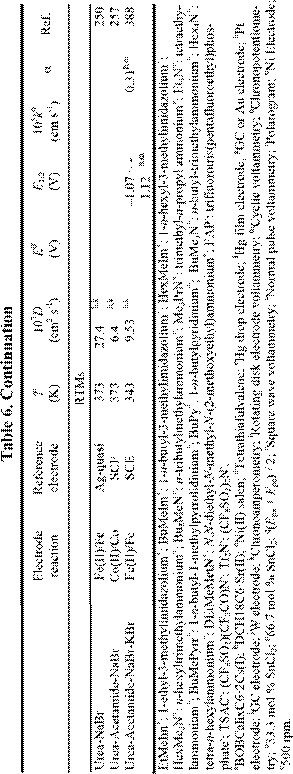
is obvious that the number of electrochemical investigations of solutes in non-chloroaluminate RTILs is relatively small, much smaller than in chloroaluminate RTILs. Although chloroaluminate RTILs have been studied for a much longer period of time, there are now more investigators who are interested in RTILs than in the past. The real reason for the paucity of electrochemical data may be simple: non-chloroaluminate RTILs are relatively inert materials and do not solvate the wide variety of materials that dissolve in the corresponding chloroaluminates. Recently, however, Chen and Hussey368 reported that ionophores can be used effectively to enhance the solubility of otherwise intractable materials, in this case, Cs(I) and Sr(II) so that they can be subjected to electrochemical studies. If this proves to be an effective method to increase the solubility of other solutes, then perhaps more electrochemical data will accrue in the near future. In this Section, we will introduce recent electrochemical research results derived from RTILs.
The application of RTILs, in particular the room-temperature haloaluminates, to surface finishing technology has a long history that spans more than half a century. In 1948, Hurley and Wier389,390 successfully used mixtures of ethylpyridinium chloride and AlBr3 as a bath for electroplating Al. At that time, the electrochemical reaction leading to the electroplating of Al had not been clarified, but it is now known to be
4 Al2X7– + 3 e– ' Al + 7 AlX4– (23)
where X is either Br or Cl. Most research is now carried out with
chloroaluminate RTILs, rather than the mixed chlorobromoaluminate systems, because those containing Br tend to undergo photodecomposition. However, in this Section, we review the progress attained to date in surface finishing technology with RTILs and RTMs. Tables 7 and 8 summarize the data about the electrodeposition of metal and alloy films from RTILs and RTMs that were available at the time this chapter was prepared. For convenience, we have classified the surface finishing techniques into three categories according to the type of solvent.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.