Although there are difficulties with rechargeable Li batteries, we expect that Li-air510 and Li-seawater511 primary batteries will achieve practical use without great difficulty. These batteries are not novel systems, but the use of hydrophobic RTILs greatly improves their performance. It is notable that the capacity of the Liair cell using EtMeIm+Tf2N– is 5360 mAh g-1 and that this performance figure exceeds that of similar cells that are based on conventional organic solvents. Figure 18 (top), which is reproduced from this article, shows changes in the cell voltage as a function of time with the measured temperature and humidity. The cell was discharged continuously for 56 days under an air atmosphere. The discharge profiles of this cell are shown in Fig. 18 (bottom) for the first 40 hours of operation. The cell performance drops off rapidly at temperatures of 253 K or less, but the performance is quite good when the operating temperature is 293 ~ 373 K. This result implies that the cell can be used effectively at temperatures of more than 373 K. In this investigation, it was also revealed that EtMeIm+ plays an important role in preventing hydrolysis of the Li negative electrode. It is likely that EtMeIm+ contributes to the formation of a stable SEI film on the Li negative electrode.
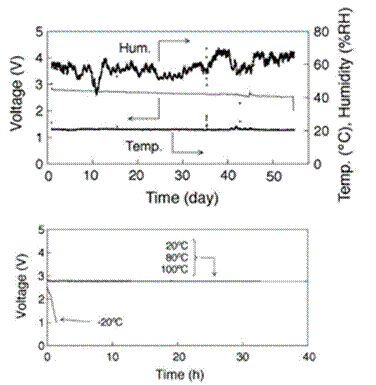
Figure 18. Top: Effect of humidity on the discharge profile in air for a Li-air cell with a EtMeIm+Tf2N- + LiTf2N (0.5 M) electrolyte. The operating current density was 0.01 mA cm-2. Bottom: Discharge curves for this cell at different temperatures under the same experimental conditions. Reproduced from Ref. 510 with permission of Elsevier Ltd.
As described at the beginning of this article, RTILs have a good chemical and electrochemical stability. Recently, the effect of radiation on the stability of several RTILs was investigated. These studies were conducted with different objectives. Some research groups were interested in utilizing RTILs as solvents for radiolytic studies, whereas others were interested in using them for the processing of nuclear materials. The radiation-induced reactions in these solvents were found to be complex,512-518 but several salts based on the n-BuMeIm+ cation were declared to be excellent solvents for the generation of radical cations and anions.513 However, the tetraalkylammonium-based RTILs such as n-Bu3MeN+Tf2N-,
tend to show the best stability toward solvated electrons,515-517 and are superior to RTILs based on dialkylimidazolium salts.518
The reprocessing of spent nuclear reactor fuel results in large volumes of liquid waste that contains many insidious by-products, notably 137Cs+ and 90Sr2+. There are many on-going efforts to develop an effective process to capture and concentrate these waste products, including liquid-liquid extraction with hydrophobic organic solvents, e.g., 1,2-dichloroethane, dichloromethane, toluene, xylene, containing macrocyclic ligands.519 Given their favorable solvent properties, it is not surprising that hydrophobic RTILs have also been investigated for this purpose.89,289,295,296,368,520-533 For example, calix[4]arenes such as calix[4]arene-bis(t-octylbenzocrown-6) (BOBCalixC6) dissolved in 1-Cn-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide ionic liquids were found to be an extremely effective extraction system for Cs+.527 Likewise, Sr2+ could be extracted into these RTILs with dicycohexano-18-crown6 (DCH18C6).89,368,520-526,528-532 Figure 19 (top) shows the selectivity ratio of Sr2+/alkali metal ions in different RTILs for two different macrocyclic ligands. This figure indicates that the Sr2+/alkali metal selectivity ratio is not good enough for DCH18C6 in these dialkylimidazolium-based RTILs to form the basis of a practical system. However, the related crown ether, N-octyl-aza-18-crown6, provides the needed selectivity as shown in Fig. 19 (bottom).526 Presently in order to improve the extraction efficiency, several approaches are being studied by modification of hydrophobic RTILs for this treatment 89,531 and through molecular dynamics simulations.533
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.