There are many reports providing electrochemical data and reaction mechanisms for the hydrogen electrode reaction in hightemperature ionic liquids.465 Compared to aqueous solvents, the mechanism in these high-temperature systems appears to be complex, but this may be a perception that is derived from a lack of knowledge and understanding. Based on the limited information that is available, the hydrogen electrode reaction is also complex in RTILs. In the basic imidazole–HTf2N system under oxygen-free conditions, the redox reaction at 0 V vs. RHE at 353 K is:466
2 HIm+ + 2 e– ' 2 Im + H2 (24)
But, under an O2 atmosphere, the reaction becomes:466,467
O2 + 4 HIm+ + 4 e– → 2 H2O + 4 Im (25)
The diffusion coefficient for HIm+ in these mixtures depends on the imidazole–HTf2N composition ratio, and the value was estimated by pulse-gradient spin-echo NMR spectroscopy to be ca. 1 ~ 6 × 10-7 cm2s-1.466
It is very important to know about the mechanism of the oxygen electrode reaction in RTILs because as mentioned above oxygen is easily reduced to O2– in anhydrous aprotic solvents (Eq. 5). Although the electrochemistry of oxygen in RTILs has been investigated by several scientists (see Section III.4), most of these experiments were carried out in very dry RTILs under an inert atmosphere with O2 and H2O < 3 ppm. There is not much information about the O2 electrode reaction in RTILs containing H+313 and/or H2O,305,309 which is the environment that is required for the operation of fuel cells. It is well known that in the presence of H+ and H2O, the reduction of O2 takes a different mechanism from Eq. (5):
O2 + 4 H+ + 4 e– → 2 H2O (26)
Thus, studies that are designed to probe and understand the O2 electrode reaction in RTILs containing H+/H2O are required before these ionic solvents can be exploited as electrolytes for use in lowtemperature fuel cells. Recently, an investigation of the O2 electrode reaction in proton conductive RTILs, was reported.468,469 The oxygen reduction reaction rate in RTILs containing HTf exhibited a higher reaction rate than with phosphoric acid and neutral RTILs.
The first reported fuel cell system based on a RTIL was of the thermally regenerative type.460 This fuel cell employs mixtures of MeEtIm+Cl- + HCl with or without added AlCl3. The pertinent cell reactions are:
Anode:
2 EtMeIm+Cl– + H2 + 2 Cl– → 2 EtMeIm+HCl2– + 2 e– (27)
Cathode:
2 HCl + 2 e– → H2 + 2 Cl– (28)
Thus, the net reaction is:
2 EtMeIm+Cl- + 2 HCl → 2 EtMeIm+HCl2– (29)
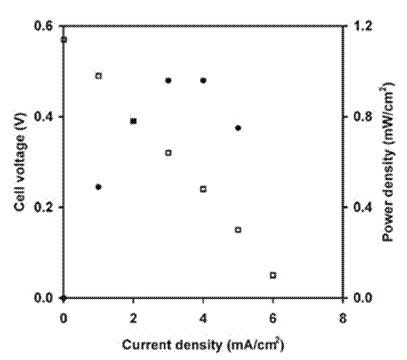
Figure 14. Variation of cell voltage (□) and power density (●) with current density for a single fuel cell using dry H2 and O2. The electrodes were Pt/C with a Pt loading of 0.926 mg cm-2. The cell was operated at 373 K. Reproduced from Ref. 475. Copyright (2006) with permission of The Royal Society of Chemistry.
The regeneration of a sufficient amount of EtMeIm+Cl- and HCl from the resulting EtMeIm+HCl2– proceeds readily when the cell is heated to more than 453 K. The actual peak open circuit voltage is 0.3 V, and the maximum power density is 0.69 mW cm-2 if the cell is heated to 383 K. Aluminum chloride is added to the electrolyte in order to lower the operating temperature.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.