Unfortunately, any practical extraction process for Cs+ and Sr2+ that is based on RTILs must include a provision for recycling the expensive extraction solvent. Recycling methods based on the electrochemical removal of the ionophore-coordinated Cs+ and Sr2+ have been investigated and found to be feasible in tetraalkylammoniumbased RTILs such as n-Bu3MeN+Tf2N-.150,289,295,368,532 The related dialkylimidazolium-based RTILs are not compatible with electrochemical recycling strategies owing to their pronounced tendency to undergo reduction at the same potential at which the ionophorecoordinated alkali metal ions are reduced. A schematic model of the proposed processing cycle for Cs+ is shown in Fig. 20.289
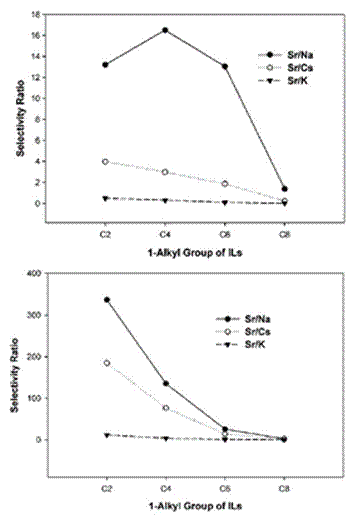
+ Figure 19. Effect of the alkyl chain length in CnMeIm Tf2N on Sr2+/Na+, Sr2+/Cs+, and Sr2+/K+ selectivities during metal cation extraction from aqueous solutions with (top) DCH18C6 (0.1 M) or (bottom) N-octyl-aza-18-crown-6 (0.1M). Reprinted with permission from Ref. 526. Copyright (2004) American Chemical Society.
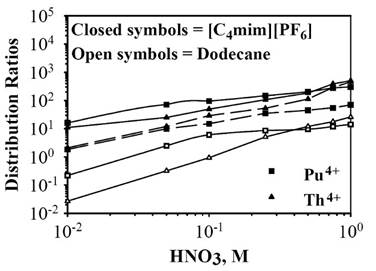
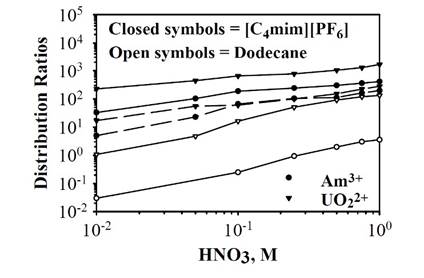
Figure 21. Distribution ratios for 241Am3+, 233UO22+, 238Pu4+, and 230 4+ + Th in the liquid/liquid extraction systems: n-BuMeIm PF6
/aqueous solution (solid symbols) or dodecane/aqueous solution (open symbols). The extracting phase is either (- - -) 0.1 M octyl(phenyl)-N,N-diisobutylcarbamoylmethyl phosphine oxide (CMPO) in n-BuMeIm+PF6- or (–––) 0.1 M CMPO + 1 M tri-nbutylphosphate (TBP) in n-BuMeIm+PF6-. Reproduced from Ref.
534 with permission of Elsevier Ltd.
The high level waste resulting from the treatment of spent nuclear reactor fuel also contains actinides that must be eliminated from the processing solution. Chemical extraction experiments have been conducted to remove actinide cations using imidazolium-based RTILs with octyl(phenyl)-N,N-diisobutylcarbamoylmethyl phosphine oxide (CMPO) and tri-n-butylphosphate (TBP).534-536 The CMPO works as an extractant, and the TBP is a phase modifier. As shown in Fig. 21, the distribution ratio increases with the HNO3 concentration independently of the solvent and obviously improves by the use of a RTIL in comparison to a conventional dodecane-based system.
There are a limited number of papers about the electrochemical behavior of actinides in the non-chloroaluminate
RTILs,363,364,385,386,537 probably because the solubility of the actinide salts is much lower in these solvents. To overcome this issue, the synthesis of actinide complexes consisting of organic cations and actinide chloride anions have been attempted.363,364,538-540 Undoubtedly, more research on this subject will appear in the near future.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.