This paper presents induction time data characterizing the lower scaling threshold limits measured with different antiscalants in a CaSO4 scaling system under various conditions. The data are shown to conform to predictions of nucleation theories and to be consistent with literature nucleation surface energies. Another important aspect of the present study is experimental verification of the assumption that the scale suppression effectiveness of an antiscalant under concentrate recycle conditions is substantially similar to that exerted under once-through flow conditions.
Despite the complexity of nucleation kinetics, literature results show that often, though not invariably, it is possible to correlate induction time data by a simple model based on the assumption that induction time J is inversely proportional to the nucleation rate J [4]:
![]() (1)
(1)
where B is a constant. The kinetics of primary nucleation, homogeneous or heterogeneous, is given by:
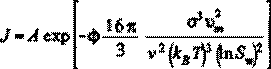 (2)
(2)
where A is a frequency factor, F is the crystal surface energy, Lm is the molar volume of the crystalline phase (7.445•10!5 m3/mol for CaSO4A 2H2O), < is the number of ions of a crystallizing salt molecules (< = 2 for CaSO4), kB is the Boltzmann’s constant (1.38A10!23 J/°K), T is the absolute temperature (°K) and Sw is the supersaturation ratio prevailing on the membrane surface. It is assumed that nuclei have a spherical shape in homogeneous nucleation. The energy barrier for heterogeneous nucleation is smaller since the nucleus surface to volume ratio is reduced by its formation on a foreign surface. This effect is represented by the parameter N, which has a value of N = 1 for homogeneous nucleation and N<1 for heterogeneous nucleation. The wetting angle of the precipitating salt governs the magnitude of the parameter N.
An expression enabling correlation of induction time with the saturation level S is obtained by combining Eqs. (1) and (2):
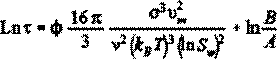 (3)
(3)
The induction period can therefore be characterized by measuring the slope of the straight line correlating Ln J with 1/(LnSw)2 and extracting the value of the surface energy parameter F. Since the magnitude of N is usually unknown, most researchers report an effective surface energy value Feff1, which embodies the parameter N as follows:
![]() (4)
(4)
Some authors erroneously apply Eq. (3) assuming that < = 1, and hence, their reported surface energy values are lower by the factor
![]() .
Denoting Feff2
as surface energy based on <
= 1, the corrected surface energy based on the actual value of < is given by
.
Denoting Feff2
as surface energy based on <
= 1, the corrected surface energy based on the actual value of < is given by
![]() (5)
(5)
The supersaturation ration Sw prevailing on the membrane surface is defined by
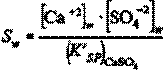 (6)
(6)
where [Ca+2]w and [SO4!2]w refer to membrane surface concentrations. These are related to the bulk solution concentrations [Ca+2] and [SO4!2] according to the concentration polarization level (CP), given by
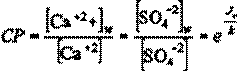 (7)
(7)
![]() where Jv
is the permeate flux and k is the mass transfer coefficient. Values of
the solubility product as a function of ionic strength and temperature were
evaluated in this study using primary literature sources [5–8], all of which
gave identical results in the range of water compositions investigated in this
work.
where Jv
is the permeate flux and k is the mass transfer coefficient. Values of
the solubility product as a function of ionic strength and temperature were
evaluated in this study using primary literature sources [5–8], all of which
gave identical results in the range of water compositions investigated in this
work.
Evaluation of the concentration polarization level CP requires estimation of the mass transfer coefficient k, either from literature correlations or by direct experimental determination. Literature correlations of the mass transfer coefficient in spiral-wound modules are uncertain due to the presence of turbulence promoters and the poorly defined hydrodynamics of flow. In this study, the mass transfer coefficient was experimentally determined in each run by a simple novel technique recently developed in our laboratory [9]. The technique is based on evaluation of the permeate flux decline induced by addition of a salt solution to an initially salt-free water feed.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.