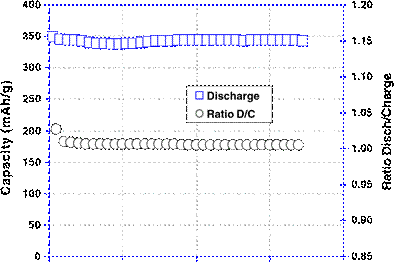
We have attempted to make full Li-ion battery with LiFePO4 as cathode and graphite as the anode, by using Py13(FSI)-0.7MLiFSI as the electrolyte to which we have added 5 wt.% of polymer. Before making the Li-ion-cell, the graphite anode was also evaluated separately in a half cell with the same electrolyte composition (IL+5 wt.% polymer). Figure 9 shows the cycling behavior at C/4 of the graphite anode. In the first few cycles, a small fading of
about 3% was noticed, and then the cell recovered with good reversible capacity stabilization at 342 mAh/g. Our choice for this amount of polymer is based on the safety aspect; the gel polymer cannot be well formed with lower than 5 wt.% of polymer in the IL-polymer mixture; hence, the desired electrode–gel interface aimed here can only be formed at polymer concentration of ≥5%. The cells have active surface area of 104 cm2 (Fig. 10) and an installed capacity of 38 mAh. The first cycles of charge–discharge at C/24 show an increase in the coulombic efficiency (CE) from 68.4% in the first cycle to 96.5% in the third cycle (Fig. 11). The low first CE in Li-ion cell is more related to the graphite anode which has in the first cycle only 80% CE. Thus, further improvements should be aimed at the graphite anode side. The reversible capacity was 113 mAh/g. After three cycles, the CE recovers to 96.5% but the battery still needs more cycles to be efficient. The power capability was evaluated for this IL-Li-ion battery based on the discharge capacity obtained at C/12. The charge was maintained at constant regime at C/6 and the discharge regime ranged from C/12 to 40 C. Stable capacity, independently of the discharge rates, was obtained below
0,0 0,1 1,0 10,0 100,0
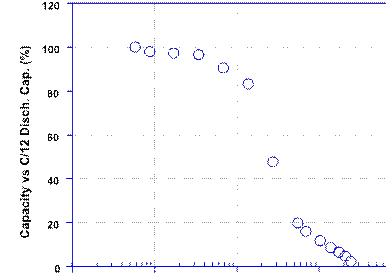
Fig. 8 Rate capability of Li-graphite cell with [Py13(FSI)–0.7 M LiFSI+5% polymer]
0 10 20 30 40
Fig. 9 Cycling behavior of Li-graphite cell with [Py13(FSI)–0.7 M LiFSI+5% polymer]
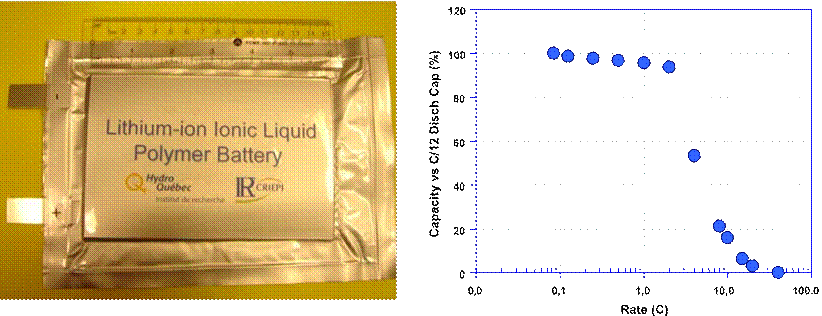 |
104 cm2
and up to 2 C rate, as shown in Fig. 12. The capacity starts decreasing at rates above 2 C, with 54% of the rated capacity still delivered at 4 C. This limitation, as we have explained in the preceding paragraphs, arises from the high concentration of ions in IL, which induce a lower free pathway of ions in the media owing to ion pairing etc. [52, 53].
On the long cycling life, the cell was cycled at the C/4 rate between 4 V and 2 V. Figure 13 shows the behavior of the discharged capacity normalized to the initial discharged capacity as function of the cycle number. After 30 cycles, the capacity of the cell fades by 10%. This capacity fade, represented by 0.33% per cycle, is slightly higher than that in the Li-ion cell with standard electrolyte. Based on the cycling data of the separate half cells (Figs. 7 and 9) we cannot attribute this fading to any of the half cells; both
Fig. 12 Rate capability of Li-ion cell with [Py13(FSI)–0.7 M LiFSI+
5% polymer]
|
Fig. 11 The first charge/discharge cycles of Li-ion cell with [Py13 Fig. 13 Cycling behavior of Li-ion cell with [Py13(FSI)–0.7 M LiFSI+ (FSI)–0.7 M LiFSI+5% polymer] 5% polymer] |
electrodes separately have shown a good stability with Py13-FSI-polymer electrolyte. But when we assemble the full Li-ion cell, the source of lithium ions is limited, thus, the inefficiency of the graphite anode half cell (80%) consumes more lithium ion than standard electrolyte to form its SEI on the graphite anode. However, this SEI layer needs few cycles till it is fully stabilized. Hence, the balancing process between anode and cathode capacities in the IL media can be one of the problems causing capacity fading. The large active surface area of (104 cm2) of the electrodes raises a technological difficulty related to the wettability of the whole active material of both electrodes with IL-polymer electrolyte. Notwithstanding this fact, we demonstrate that a Li-ion battery having LiFePO4 and graphite as the cathode and anode, respectively, with an ionic liquid as the electrolyte is achievable. Some applications where the high power is not the requirement can benefit from this technology. However, many technical parameters such as the electrode porosity, the separator porosity and the wetting process should be further improved during further technical development.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.