Li-ion (graphite/LiCoO2) [31] in DEME-FSI with 10 wt.% VC additive. Other work has examined high capacity silicon anodes using methyl-1-propylperidinium-TFSI known as PP13-TFSI silicon [32], and, SiOx mixed with carbon using Py13 based IL [33].
Recently Aurbach et al. found that intercalation in the graphite anode occurs simultaneously with IL-cations and Li+ cations at potential ∼500 mV and even below 300 mV, by using In-Situ Raman spectroscopy analysis [34]. Ishikawa et al. demonstrated that it is possible to intercalate Li+-ion in the graphite anode without any additives. By using an appropriate anion like bis(fluorosulfonyl)imide (FSI) [35], we have confirmed this result [36]. We have found that with the same IL-cations by changing from TFSI to FSI anion the electrochemical properties can be vastly improved. To understand the FSI-anion effect on the graphite intercalation, recently some work was done on XPS characterization and it revealed that the surface layer on the graphite in both systems, EMI-TFSI and EMI-FSI, are chemically similar. This effect is still not well understood; we think further work should be addressed to this point. From the safety aspect, in our previous work [37], we found that the reactivity of some ionic liquids is higher than others; ILs with EMI cations are worse from safety point of view than those with BMMI, Py13, PP14, and TMBA. In order to work on a future battery we tried to combine the safe materials.
Following our previous work mainly on the anode, briefly recapitulated below, we have investigated here the LiFePO4 cathode side, and, then the full Li-ion battery with an ionic liquid. Thus we present work here on a safe battery configuration containing carbon anode and LiFePO4 cathode, and, Py13-FSI with LiFSI as salt additive plus gel polymer together constituting the electrolyte. Some effects of the polymer addition to the battery system are also examined.
The solvents chosen for this work are two ionic liquids; 1ethyl-3-methylimidazolium-bis(fluorsulfonyl)imide (EMIFSI), N-methyl-N-propylpyrrolidinium-bis(fluorsulfonyl) imide (Py13-FSI) based on FSI anion (Fig. 1), produced by Dai-Ichi Kogyo, Seiyaku Co., Ltd, Japan (DKS). These ionic liquids (IL) contain less than 10 ppm (w/w) of moisture and less than 2 ppm (w/w) of halide and alkali metal-ion impurities. Our interest in these ILs is related to the FSI anion when combined with high conductivity and lower viscosity cations such EMI; also on the use of wider potential window IL (Py13). Two organic electrolytes were also evaluated; the standard electrolyte ethylene carbonate/ diethylcarbonate EC/DEC–1 M LiPF6 (UB, Japan) was used as the reference. The second organic electrolyte was EC-DEC, in which we have dissolved 1 M of lithium bis (fluorosulfonyl)imide (LiFSI) (from DKS, Japan) was used.
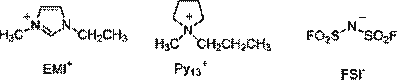
A stainless-steel coin type cell was used for this study. The anode was a composite electrode containing 5 wt.% of PVDF (Kureha, Japan), 93% of natural graphite (HydroQuébec) and very small amount of VGCF (vapor-growth carbon fiber) fiber (Showa-Denko, Japan). The cathode was constituted by 12% PVDF (Kruha, Japan), 82% of LiFePO4 (Phostech-lithium, Canada) carbon coated, 3 wt.% of carbon black and very small amount of VGCF. We wanted to get the highest salt concentration but without going as high as it will cause some ion-pair formation and, thus, lower mobility and, thence, lower conductivity. Based on this, the electrolyte was prepared by adding 0.7 M LiFSI in both Py13-FSI, and, EMI-FSI ionic liquids. For the cell assembly, the Cellgard (3501) was soaked into the organic solvent or the ionic liquid electrolyte, under vacuum at 60 °C. The active surface area was 2 cm2. The cell fabrication was performed in the glove box with lithium metal as the counter electrode. The electrochemical measurements were performed by using VMP-cycler (Biologic, France). The first formation cycles were obtained at constant charge–discharge at C/24. The electrodes were evaluated for power performance with the C-rate test, by varying the discharge current from C/12 to 40 C. The conductivity measurements were done by using a model CM-30R conductivity meter (from DKK-TOA Corp. Japan). The viscosity was measured by means of a MCR-30 viscometer (Anton-Paar, USA). Owing to its better safety profile, Py13(FSI) was considered for further study in this work.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.