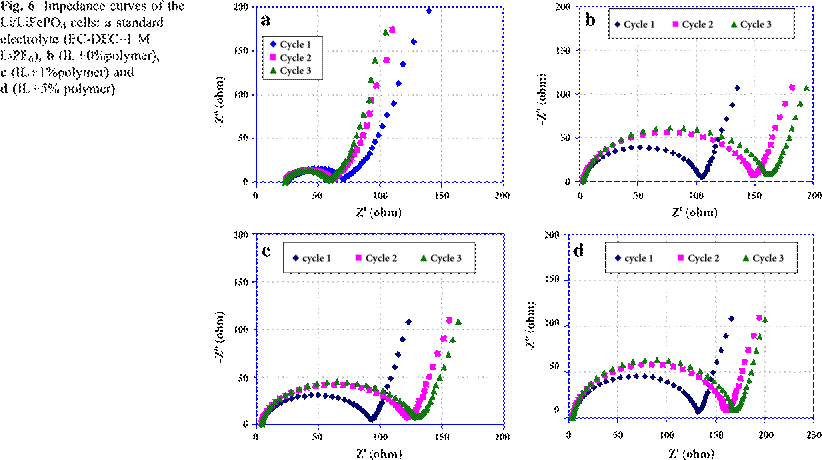
The diffusion resistance is plotted in Fig. 5b as a function of %DoD with different amounts of polymer in the IL. The same behavior of Rd may be noted at different states of charge with or without polymer additions. The highest value of Rd is obtained with the monophase iron phosphate; at 0% DoD with LiFeO4 and at 100% DoD with FePO4 phase. The diffusion process is easier when the diphase LiFePO4–FePO4 coexists, and particularly in the range of 50–70% DoD.
 |
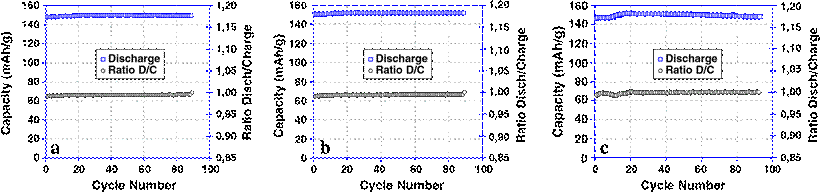
The cells were cycled at C/4 rate for a long cycle life aging (Fig. 7), the reversible capacity was found stable in all cases at 149, 152, and 148 mAh/g respectively for (a) IL, (b) (IL+1 wt.% polymer) and (c) (IL+5 wt.% polymer). The coulombic efficiency remains constant during the cycling life with 99.6% for all cells. However, we noted an abnormal behavior with 5% polymer cell. In the first 20 cycles, the capacity increases and the coulombic efficiency fluctuates before the cell reaches the stable condition. Moreover, this result confirms the stabilization of both electrode interfaces of LiFePO4 and lithium electrodes with IL which probably suppresses the dendrite formation on the lithium metal. The rate capability of the cell, Li/(IL+5 wt.% polymer)/LiFePO4, is shown in Fig. 8. The cell delivers the full capacity until around C/2 rate, and then the discharged capacity starts decreasing to reach 82% at 2 C. At 4 C rate, the capacity dropped sharply to 47% and continues dropping as the discharge rate is increased. This drop in
 |
the capacity above 2 C rate perhaps indicates that the limiting process lies in the ionic liquid: it is well-known that a very high concentration of ions in the molten salt can lead to “over-population” of ions that can cause phenomena such as ion-pair formation and “salting out”, in the sense that ion–ion distance is short thus disabling the “free” ion to make full contribution to the conduction [52, 53]. Such a situation, of course, does not exist in the standard organic electrolyte [36] in which the solvent molecules are abundantly available to solvate the lithium ions which then contribute to conduction.
Li-ion cell with IL and polymer gel
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.