Figure 3.a shows that the interface
resistance (Ri), for the standard electrolyte, increases at 10% DoD (Depth of
Discharge) then decreases with increase of the DoD till for fully delithiated
LiFePO4 it reaches 40 Ω.
The highest value of Ri was 100 Ω,
found at 10% DoD, when 10% of the lithium is removed from the LiFePO4 structure.
The cells having an ionic liquid as the electrolyte show that Ri increases at
both low and high %DoD, contrary to the cell with standard electrolyte. After
delithiation of LiFePO4 up to 10%, the Ri increases from 150 Ω to 165 Ω, and then stays stable at
around 150 Ω when more
LiFePO4 is transformed to FePO4. When LiFePO4 is
completely transformed to the FePO4, phase Ri decreased to lower
values to finally reach 105 Ω.
Since the high viscosity of the IL causes a problem of cathode wettability,
compared to the conventional electrolyte, higher Ri is obtained with cells
having IL as the electrolyte. Contrary to the conventional electrolyte, the
average Ri behavior is maintained constant until 90% DoD after which an
increase in Ri occurs. When all the iron phosphate material is delithiated and
the monophase FePO4 is the dominant phase, the Ri decreases to a
level lower than the Ri of the fully lithiated monophase LiFePO4 (0%
DoD).
In the diffusion resistance part (Rd; Fig. 3b), Rd is higher in the conventional electrolyte than with the IL cell. Both electrolytes show a similar behavior; a decrease of Rd when the diphase LiFePO4–FePO4 coexists. Higher Rd was obtained with both monophase states of iron phosphate; LiFePO4 at 0% DoD and FePO4 at 100%DoD.
2. Ionic liquid with polymer additive
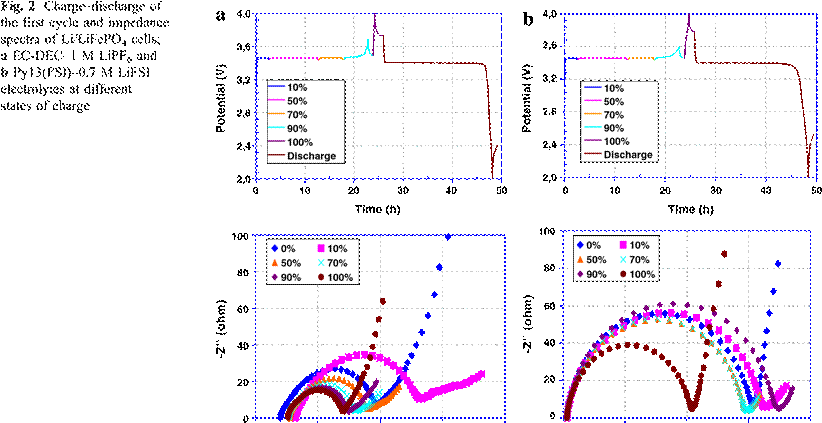
The effect of polymer addition to the IL was analyzed by in situ impedance spectroscopy. Figure 4 shows the charge– discharge of the first cycle and the associated impedance spectra at different states of charge of the Li/FePO4 cells at (a) IL (Py13(FSI)-LiFSI), (b) IL+1% polymer and (c) IL+
5% polymer; the first-coulombic efficiency was found to be 97%, 100%, 99%, respectively, with these cell configurations. The reversible capacity was close to159 mAh/g for all cells which is comparable to a cell with a standard electrolyte.
|
polymer, b 1% polymer c 5% polymer additive |
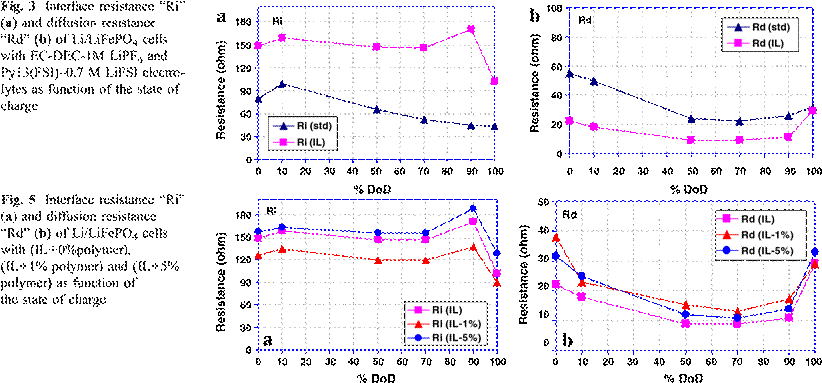
Figure 5a, shows the interface resistance Ri
with IL cells, as well as for those to which 1% or 5% by weight of polymer is
added. The Ri is increased when 5% polymer is added to the IL cell. However,
when only 1% of polymer is added, Ri is lower than in the cell with IL alone at
different level of %DoD. The average Ri values are maintained constant during
the LiFePO4–FePO4
transformation with a higher Ri value at 90% DoD followed by lower Ri at
monophase FePO4. This behavior of the Ri variation is still present,
independently of polymer addition. Therefore, from these results, we can assume
that the addition of small amount of polymer (1%) improves the interface of the
cathode in the cells with IL. This small amount of polymer can play an
important role in making a stable solid electrolyte interphase layer (SEI),
better than when the IL alone is used. The addition of more polymer in the IL
cell (5%) shows a contrary effect with an increase of the interface resistance
even higher than that for the IL without additive. We may infer that, the
addition of 1% polymer stabilizes the SEI, and then reduces the interface
resistance by forming a thin passivation film. Higher polymer content in the IL
forms a thicker resistive layer which can lead to the increase in the Ri. The
polymer would be expected to form a stable thin layer on both anode and cathode
which is permeable to Li+ ions. In the half cells, the polymer would
passivate the lithium metal and decrease its reactivity, and reduce surface
energy.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.