In RO desalting, as product water recovery level is increased, the retentate stream is concentrated relative to the feed by a concentration factor (CF), which can be estimated from:
![]() C 1
C 1
CF(1) 1 − W
in which CC and CF are the retentate (concentrate) and feed concentrations, respectively, RS is the nominal salt rejection (RS =1−CP/CF, where CP is the permeate concentration), and
RW isthefractionalproductwaterrecovery(RW =QP/QF,where QP and QF are the permeate and feed volumetric flow rates, respectively). At high salt rejection, CF rises rapidly with increased recovery as recovery increases above ∼80%. For example, for desalting at recovery levels of 83% and 95% (assuming Rs ∼ 0.98), the retentate stream will be concentrated relativetothefeedbyaboutafactorof5.8and19.6,respectively. As recovery increases, the retentate stream is concentrated and thus the potential for mineral salts to precipitate out of solution also increases. As an example, thermodynamic calculations of the saturation indices (SI) for the major mineral salts of concern for the concentrate produced from CR water at 83% recovery is shown in Fig. 1. The supersaturation index, which expresses the level of saturation of a solution with respect to the various mineral salts, is defined as
SIx![]() (2)
(2)
where IAP is the ion activity product and Ksp,x is the solubility product for mineral salt x. A saturation index greater than unity signifies that the solubility limit for the mineral
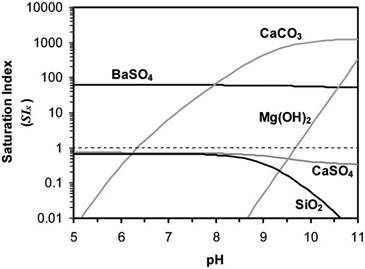
Fig. 1. Saturation indices of BaSO4, CaCO3, CaSO4, SiO2, and magnesium hydroxide as a function of pH for CR water concentrate produced from RO desalting at 83% water recovery at 98% nominal salt rejection, calculated based on average raw water quality of CR water (Table 1) at concentration factor of 5.8 and at 25◦C.
salt is exceeded, thus indicating a potential membrane scaling problem. As suggested by the results shown in Fig. 1, the primary scalants of concern for desalting of up to about 83% are BaSO4 and CaCO3. With further water recovery from the PRO concentrate, CaSO4 and silica (SiO2) would be expected to play increasingly important roles in limiting water recovery.
The possible applications of various precipitation processes forthetreatmentofPROconcentratetoalleviatemembranemineral scaling and thus achieve high recovery have been discussed in a number of scoping studies using a variety of conceptual process schemes [2–12]. Although recovery levels in excess of 90% have been reported, some of these processes were plagued by fouling, corrosion, and premature membrane damage [7,9]. More recently, Rahardianto et al. [12] demonstrated that up to 98% total system water recovery was possible for CRW RO treatment at the bench scale using ICD, supplemented with the use of make-up antiscalant for secondary RO desalting. ICD was effective in reducing the concentrations of Ca2+ and other membrane scaling precursors from the PRO concentrate below saturation or to a metastable supersaturation range with respect to several problematic mineral salts, so as to allow further RO desalting of this concentrate stream. As suggested by the solubility calculations presented in Fig. 1, pH adjustment is another possible approach that can be applied to mitigate scale formation of some potential scalants (e.g., CaCO3, Mg(OH)2 and SiO2), though at differing pH ranges. However, the solubility of CaSO4 and BaSO4 are relatively independent of pH. Therefore, further desalting of the above PRO concentrate, even at low pH, would be a challenge since the solution is nearly saturated with respect to CaSO4 and SiO2 and oversaturated with respect to BaSO4. Chemical demineralization of the PRO concentrate via ICD, however, should enable further desalting to achieve higher recovery. Rahardianto et al. [12] developed an operational strategy for the ICD process whereby the minimum required level of Ca2+ removal, to achieve a specific product water recovery by secondary RO desalting, was estimated by
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.