VMNP can be caused by a basic disease of the newborn interfering with renal perfusion or can be secondary to activation of some of the vasoregulatory systems. For example, hypotension increases the activity of the RAAS and the sympathetic nervous system, hypoxemia stimulates ET, ANP, and PG release, whereas septicemia raises ET and NO activity. The interactions between these regulatory forces are rather complex. To complicate matters, several of the vasoconstrictor and/or vasodilatory factors differ in their effects on the systemic and (intra) renal circulation. A factor having a beneficial effect on the systemic circulation may even have harmful renal consequences. An example is the activation of All in hy-potensive states. All stimulation results in increased MAP but may also decrease renal perfusion. The therapeutic use of the PG synthesis inhibitor indomethacin represents another example. By closing a PDA, indo-
Hypoxemia
The second most-frequent condition leading to neonatal VMNP is perinatal hypoxemia or asphyxia, generally in the course of a severe respiratory distress syndrome (RDS) [2, 35]. Hypoxemia by itself reduces RBF and GFR. In a recent study, 61% of severely asphyxiated newborns developed ARF [36]. Hypotension, hypovolemia, activation of the RAAS and the intrarenal adenosine system, as well as stimulation of catecholamines and increased vasopressin release, are some of the factors observed in human neonates or neonatal animal models with RDS-induced hypoxia [37-40]. All of these factors can result in disturbances of glomerular hemodynamics (Fig. 1). Hypercapnic acidosis, another consequence of a variety of respiratory disorders, including neonatal RDS, can cause renal vasoconstriction, thus contributing to the overall renal dysfunction seen in severe asphyxia [41].
Septicemia
Septicemia is also an important cause of neonatal ARF. This form of vasomotor ARF is generally part of multi-organ failure with renal hypoperfusion - secondary to systemic hypotension - and activation of several vasoac-tive mediators [42]. These vasoactive mediators, such as the RAAS, ET, the renal KK-kinin system, and throm-boxane (TXA2), are probably more important than the hypoperfusion, and are potential future targets for specific therapeutic interventions [43].
230
|
|
|
Salt retention Water retention |
Fig. 1 The main pathways of renal dysfunction in respiratory distress. Mechanical ventilation as well as the hypoxemia-in-duced activation of vasoactive factors contribute to the development of systemic hypotension and hypovolemia (RAS renin-angiotensin system, ADH antidiuretic hormone, GFR glomerular filtration rate)
MAP V GFR RPF FF RVR
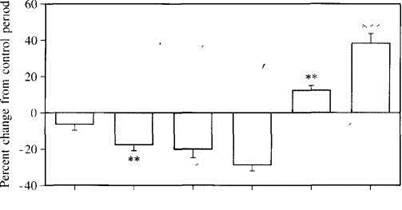
Fig. 2 Effects of a 2°C decrease in body temperature of normal, newborn New Zealand white rabbits on mean arterial pressure (MAP), urine flow rate (V), GFR, renal plasma flow (RPF), filtration fraction (FF), and renal vascular resistance (RVR). **P<0.01, ***P<0.001
Hypothermia
In newborn rabbits mild hypothermia (a decrease of 2°C) causes pronounced renal vasoconstriction, accompanied by a fall in GFR (Fig. 2) [44]. The body temperature of the neonate is probably pivotal for the maintenance of normal renal function.
Iatrogenic causes
A variety of therapeutic interventions in neonates, such as artificial ventilation, umbilical artery/vein canalization, and the administration of vasoactive or nephrotoxic drugs, may have harmful renal consequences [45, 46J.
Ventilation
Ventilation of human neonates and newborn animals with continuous positive airway pressure has deleterious effects on renal function due to decreased venous return and low cardiac output, increased renal sympathetic nervous activity, and high serum vasopressin levels [47, 48J (Fig. 1). Hyperoxemia does not seem to cause functional renal disturbances in the newborn rabbit model [49].
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.