the possibilities for future therapeutic strategies - prevention as well as treatment - that hopefully one day will change the outlook of ARF.
Prevention
The search for mechanisms to prevent ARF has followed two main approaches. In the first, investigators tried to define and locate the mediators that lead to renal hypo-perfusion and a reduction in GFR. The second attempts to clarify the glomerular and tubular intracellular events occurring during the development of renal failure. The latter approach is mainly looking for new ways to treat established renal cellular injury, whereas the first offers hope for the prevention of VMNP and secondary renal cellular damage. From a clinical point of view, the cellular approach seems to offer the greatest promise, since clinicians generally encounter patients with established ARF. However the neonatologist is in the unique position of being able to identify very early those infants who are at high risk of developing ARF.
Vasomotor disturbances
Once intrinsic renal functional changes have developed, volume resuscitation or additional attempts to prevent hypoperfusion are without effect and may even be harmful. The major mechanisms involved in the development of VMNP, elucidated during the last decade, have been summarized above. Counteracting these mechanisms has to date only been tried in experimental animals, and has failed to provide answers for clinical use in humans. The main humoral candidates for mediating renal vasoconstriction in VMNP are adenosine, ET, All, and TXA2.
Adenosine is a degradation product of ATP hydrolysis, and is increased in renal hypoxia or ischemia. Vascular beds usually respond to adenosine with vasodilation. In the glomerular circulation, however, adenosine exerts a dual role when the RAAS is activated, and induces efferent vasodilation and afferent vasoconstriction (Fig. 4).
Adenosine antagonists are able to reverse the intrarenal vasoconstrictor state of ARF [37]. Low-dose theoph-ylline (TP) (0.5-1 mg/kg), a xanthine derivative with strong adenosine antagonistic properties, prevents hyp-oxemia-induced renal insufficiency in both adult and newborn rabbits [113]. Low-dose TP (1 mg/kg) also produced renal improvement in newborns with RDS [114]. In the latter study, TP was given to neonates with FM- i resistant renal dysfunction and improved diuresis, with ' an increase in creatinine clearance. Higher doses of the drug (2-5 mg/kg) induced diuresis and increased GFR in newborns with idiopathic neonatal apnea [115]. TP also prevented the contrast media-induced renal dysfunction in humans [116]. Enprofylline, a xanthine derivative devoid of adenosine antagonistic properties, has no effect on the hypoxemic renal dysfunction of newborn rabbits [37]. This clearly shows that adenosine antagonism is the main mediating mechanism of the action of TP.
235
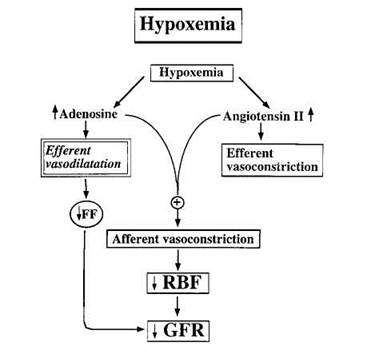
Fig. 4 Effects of intrarenal adenosine and angiotensin II on the glomerular (micro)circulation, GFR, and renal blood flow (RBF) in renal hypoxia
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.