The excel unit operation is a fast and easy way to build simulations even when there is no appropriate unit operation in CHEMCAD. One such case is fractional crystallization.
Fractional Crystallization yields high purity product with lower energy requirements than distillation. It is used primarily to separate isomers with high melting points such as mixtures of xylene.
While we do have a crystallizer unit, in it the solubility function is a function of temperature only. In fractional crystallization, solubility is a function of solution composition and temperature. To illustrate the use of this approach I looked at two systems: Xylenes in Benzene and Acetic Acid in Water.
The literature has shown that the solubility of a component (Xi) in solution may be predicted by the following equation:
Xi= (Exp((Hfusion / (1.987 * Temp)) * ((Temp / Tmelt(i)) - 1))) / ActCoeff(i)
Where
Xi=Solubility of component i
HFusion=Heat of fusion (Melting)
Temp=System Temperature
Tmelt(i)=Melting point of component i
ActCeoff(i)=Activity coefficient of component i
Our unitop (unit operation) must incorporate this function to determine the solubility of each component, and then complete a mass and energy balance around the unitop to determine the outlet composition.
This unitop presented us with a number of challenges. First, it requires data on heat of fusion and melting point for pure components, properties CHEMCAD does not currently track.. Also, components in CHEMCAD are either solid or not, they are never liquids in equilibrium with a solid phase.
We decided to add the melting point and heat of fusion data to the dialog screen. While this information is really component based, it is not possible to add new categories of data to our component databank using Excel.
To be able to handle solid in equilibrium with a liquid, we decided to define the freezing process as a reaction: liquid water->solid ice + heat. We then added new user components to represent the solid phases. So our component list for an acetic acid/water system would include:
Shown below is the dialog created for this new unitoperation. The dialog screen is filled out for an Acetic Acid/ Water system.
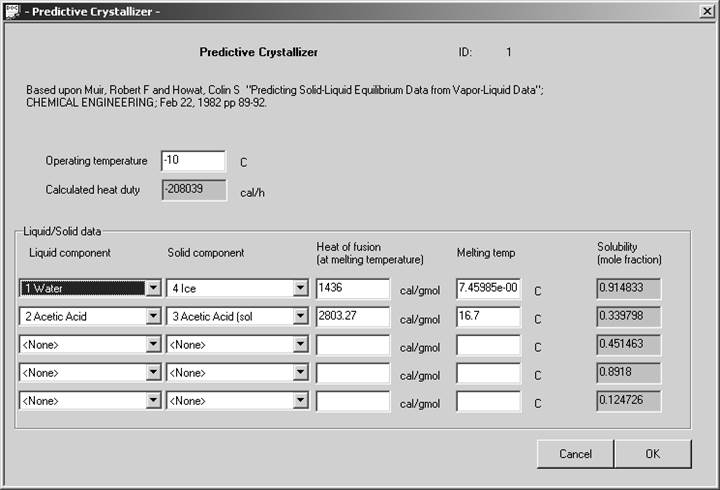
The performance of this unit is remarkably close to experimental data. Two systems were considered for testing, a para-Xylene /meta Xylene mixture and a freezing point calculation for Water/Acetic acid.
In the Acetic Acid/Water case, the freezing point data is very close to measured data:
Graph AceticAcid + Xylenes
For more information, or for a copy of the predictive crystallizer example, please see our website
Minimum
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.