The plane of the carboxyl group (O![]() C
C![]() O plane) is almost coplanar with the carboxyl side polymethylene
chains for both molecules A and B. In this case, the
C(3)
O plane) is almost coplanar with the carboxyl side polymethylene
chains for both molecules A and B. In this case, the
C(3)![]() C(2)
C(2)![]() C(1)
C(1)![]() O group takes a cis or trans arrangement about the C(1)
O group takes a cis or trans arrangement about the C(1)![]() C(2) bond as shown in Figure 8. Judging from the C
C(2) bond as shown in Figure 8. Judging from the C![]() O
bond lengths and the torsion angles about C(1)
O
bond lengths and the torsion angles about C(1)![]() C(2),
the C(1)
C(2),
the C(1)![]() O(2) group should be assigned to the C
O(2) group should be assigned to the C![]() O group and the C(3)
O group and the C(3)![]() C(2)
C(2)![]() C(1)
C(1)![]() O(2) portion takes the cis conformation in both molecules A and B.
Some polymorphs of fatty acids show cis−trans tautomerism through simultaneous
transfer of two hydrogen atoms.32 It is considered that the hydrogen-bonded O···O distance is
closely related to the occurrence of the cis−trans tautomerism.33 The O···O distance is 2.634 Å for molecule A and 2.654 Å for
molecule B. The former is rather smaller than the value found in the polymorphs
where cis−trans tautomerism does not occur (2.67−2.68 Å).30,34 There is a possibility of tautomerism at least for molecule A.
O(2) portion takes the cis conformation in both molecules A and B.
Some polymorphs of fatty acids show cis−trans tautomerism through simultaneous
transfer of two hydrogen atoms.32 It is considered that the hydrogen-bonded O···O distance is
closely related to the occurrence of the cis−trans tautomerism.33 The O···O distance is 2.634 Å for molecule A and 2.654 Å for
molecule B. The former is rather smaller than the value found in the polymorphs
where cis−trans tautomerism does not occur (2.67−2.68 Å).30,34 There is a possibility of tautomerism at least for molecule A.
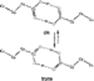
Figure 8 Cis and trans conformations of C(3)![]() C(2)
C(2)![]() C(1)
C(1)![]() O
group.
O
group.
·
o Top of Page
o Introduction
o Experimental Section
o Results
o Discussion
o Supporting Information Available
Discussion
1. Structure of the (001) Crystal Face. As described in the previous section, the β phase of oleic acid has a characteristic interdigitated structure. The intensity distribution of the (00l) reflections reflects this structure. Long-chain compounds usually exhibit a series of intense (00l) reflections. In this case, the strong intensities of the (00l) reflections of low orders of l are ascribed to the electron deficiency at the lamellar interface consisting of methyl terminals.35 However, there is no such electron deficiency at the lamellar interfaces of oleic acid β, where both methyl groups and carboxyl groups are present.
Triglycerides containing a cis-unsaturated chain in the second position form a three-layered structure. It is assumed that cis-unsaturated acyl chains form a nonsegregated structure in the centered layer.21-23 We consider that the structure of the β phase is helpful for the model building for the polymorphic phases of such mixed saturated and unsaturated triglycerides.
On the basis of this interdigitated structure, it could be inferred that the (001) crystal face of the β phase also forms a characteristic structure. The hydrogen bonding of fatty acids is so strong that it is natural that the molecules at the surface also take a dimer structure. If this expectation is correct, the population of molecules at the first surface layer is half as much as that in the inner layers. Furthermore, it can also be expected that there are two types of surface structures; one is formed with dimers of molecule A and the other with dimers of molecule B. The incidence of the two types of surfaces may depend on their surface energy. Because of the low density, the structure of the first layer probably exhibits a strong temperature dependence. At low temperatures the molecules may tend to form a dense structure, taking advantage of an increase of cohesive energy, and near the melting point the molecules may move to change the packing into a looser structure favoring higher entropy.
2. Possible Crystal Structure of β2. Although the crystal structure of the β2 phase has not been determined yet, we inferred that the β2 phase has a strong structural resemblance to the β1 phase on the basis of the following similarities in X-ray diffraction patterns (Figure 4).
(1) There is no intense reflection due to a lamellar structure in the low-angle region of 2θ < 10°.
(2) The positions of the weak reflections due to long spacings are almost the same as those of the β1 phase. For instance, the (002) reflection was observed at about 5° in both β1 and β2.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.