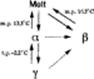
Figure 1 Polymorphism of oleic acid. Solid and broken lines represent reversible and irreversible processes, respectively.
The β phase is unique in the polymorphism of cis-monounsaturated fatty acids: in contrast to the α and γ phases, it has been found only in oleic acid and does not show an order−disorder type solid-state phase transition like the γ → α transition. Only irreversible transitions to the β phase have been confirmed: a very slow solid-state irreversible transition from α and solution-mediated transitions from α and γ. The crystallization behavior of the β phase is also unique.14,15 The growth rate is quite slow compared with the other polymorphic phases of cis-monounsaturated fatty acids. In melt crystallization at a supercooling of 0.5 °C, the growth rate of the β phase is ca. 105 times slower than that of the α phase. Such a remarkable difference among polymorphic phases has not been reported so far.
The powder X-ray reflection pattern of the β phase suggests a peculiar crystal structure. Polymorphic phases of fatty acids (except the A1 and A-super forms of lauric acid) form a layered structure whose lamellar interface consists of methyl terminals. This layered structure makes a clear periodical contrast of electron density along the lamellar stacking direction: a low-density region of methyl terminals and a high-density region of dimerizing carboxyl terminals. The clear contrast results in intense reflections assigned to long spacings for 2θ < 10°. Indeed, the α and γ phases of oleic acid exhibit intense reflections of long spacings. However, only several weak reflections are observed in the same range for the β phase, which cannot be interpreted with the layered structure where methyl and carboxyl groups are segregated from each other.
Experimental results described above indicate that the difference in the aggregation state changes the physicochemical properties remarkably. In this study, we investigate the crystallization process of the β phase and its crystal structure. At first, we describe that the β phase can be classified into two modifications: stable β1 and metastable β2. Next we show the unique molecular conformation and aggregation state in the crystal structure of the β1 phase. We also discuss the factors affecting the physicochemical properties specific to the β phase.
·
o Top of Page
o Introduction
o Experimental Section
o Results
o Discussion
o Supporting Information Available
Experimental Section
Materials. Ultrapure oleic acid (purity > 99.9%) was supplied from NOF Corporation. Acetonitrile (>99% purity, Nakarai Tesque) was employed as solvent for growing single crystals, which were used for X-ray crystal structure analysis.
Microscopic Observation. Crystal growth of oleic acid was observed with a transmission type Normarsky interference contrast microscopy (Olympus IMT2-NIC). The temperature was measured with a copper−constantan thermocouple. The temperature of a glass cell for microscopic observation was controlled within ±0.05 °C by circulating water from a thermostat (Tokyo-Rika).
For the observation of crystals of β that spontaneously occur, a melt of β was kept at 17.0 °C about 10 min and cooled to 10−11 °C. Usually, the crystallization of β started within 1 h. Then the specimen was heated to a certain temperature for morphology observation (parts a−c of Figures 2).
A small seed crystal of β was also used (Figures 2d and 3). At first a single crystal β was melted to several microns at 16.3 °C, and then it was cooled to a certain temperature.
The rate of temperature change for the above procedures was 0.3−0.5 °C/min.
DSC Measurement. DSC (differential scanning calorimeter) measurement was performed with Seiko Denshi DSC system (Model 5200). Samples (10 mg) were sealed in aluminum pans. The DSC data were taken between −20 and 30 °C with a heating rate of 2.0 °C/min.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.