BIOPHYSICS OF A CELL
1. Plasma membrane
A cell’s content is delineated from the world around it by a flexible sheet of fatty material called the plasma membrane or cell membrane. The membrane regulates a constant traffic of materials into and out of the cell, allowing water, ions, and certain organic molecules to permeate, or pass through, the boundary and allowing toxic or useless by-products of cellular metabolism to exit, at the same time keeping unneeded materials from entering and useful cell contents from oozing out. Besides its barrier activities, the plasma membrane also receives and generates signals that are an important part of the communication and coordination between cells.
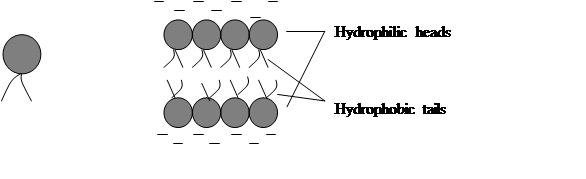
The matrix of the membrane-that is, its background material-is formed by the group of fatty organic compounds called phospholipids. Each molecule of this type has a head and tail. When such compounds are surrounded by water, they align in characteristic two-layered sheet with hydrophobic (“water-fearing”) tails pointing inward, the hydrophilic (“water loving”) heads pointing outward, and water excluded from middle (fig.1)
Water
 |
Water
Fig.1. Two-layer phospholipid sheet
Since the cytoplasm is watery and since cells must be bathed in fluid on the outside, the phospholipids naturally assume this two-tiered configuration. And it is this lipid bilayer that is largely responsible for the membrane’s barrier function.
Cell biologists have show that the cell membrane is a selectively permeable lipid bilayer (one that allows passage of certain materials but not others) studded with proteins that regulate the flow of materials into and out of the cell. This concept is the fluid mosaic model of membrane structure. The term mosaic refers to the fact that the proteins are scattered about as in a tile mosaic and can move about the fluid plane like floating icebergs.
2. Transport tasks in the dynamic cell
The cell’s inner pool is called the intracellular fluid, and the materials for maintaining that pool are taken up from the fluid outside the cell. A unicellular organism is almost always surrounded by fresh water or seawater or by the body fluids of another organism that it inhabits. In a multicellular organism, the fluid outside the cell is usually extracellular fluid, which fills all the spaces around and between cells. Materials can move back and forth between the intracellular fluid, extracellular fluid, and blood across the membrane barriers that separate them.
Such movements are governed in part by gradients, differences in the concentration or pressure of materials in one part of a contained area compared to another. Materials-ions, water, amino-acids, and so on- tend to move against concentration gradients, that is, from region of higher concentration (or pressure) to region of lower concentration (or pressure). When a substance enters or leaves a cell by moving against such a gradient, the process is called passive transport: the material moves without energy expenditure by the cell.
The tendency of materials to move from areas of high concentration to areas of low concentration is called diffusion. When the concentrations on both sides are equal, equilibrium is reached, and the same number of molecules moves in both directions at the same rate, and so no further change occurs. Most materials enter or leave cell via this process of simple diffusion.
For some large or electrically changed molecules, this problem is overcome by means of a special type of diffusion, which is still passive but involves carrier molecules that allow certain substance like glucose and the waste product urea to pass through a membrane while many other molecules cannot move across.
The movement of water, through a selectively permeable membrane (such as a cell’s plasma membrane) against gradients of concentration or pressure has its own name: osmosis.
Examples of osmosis:
Water moves from a region where it is present in high concentration or pressure to one where it is present in low concentration or pressure. An isotonic solution (“iso” means “same”, and “tonic” refers to osmotic properties) outside the cell has same osmotic properties as the fluid in the cell’s interiors and [H2O]i= [H2O]e fig.2.
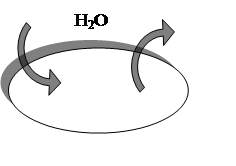 |
Fig.2. Solutes concentration equal inside and outside red blood cell, [H2O]i= [H2O]e
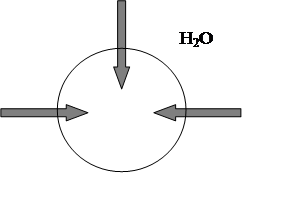 In pure water, the fluid outside the cells would be hypotonic(“hypo” means “low”) compared to fluid inside each cell, since distilled
water has a vastly lower concentration of ions, proteins, and other solutes
than the cell’s cytoplasm. And water will tend to move into the cell by osmosis
and [H2O]i<[H2O]e fig.3.
In pure water, the fluid outside the cells would be hypotonic(“hypo” means “low”) compared to fluid inside each cell, since distilled
water has a vastly lower concentration of ions, proteins, and other solutes
than the cell’s cytoplasm. And water will tend to move into the cell by osmosis
and [H2O]i<[H2O]e fig.3.
Fig.3. Solutes less concentrated outside red blood cell, [H2O]i<[H2O]e
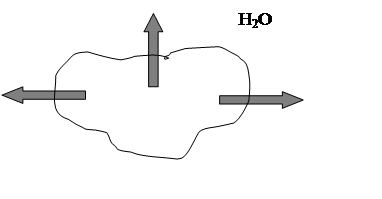 A hypertonic solution-one with a higher concentration
of salts, sugars, proteins, and other solutes than the red blood cell’s
intracellular fluid. And water would move outward by osmosis and [H2O]i>[H2O]e fig.4.
A hypertonic solution-one with a higher concentration
of salts, sugars, proteins, and other solutes than the red blood cell’s
intracellular fluid. And water would move outward by osmosis and [H2O]i>[H2O]e fig.4.
Fig.4. Solutes more concentrates outside red blood cell, [H2O]i>[H2O]e
3. Active transport
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.