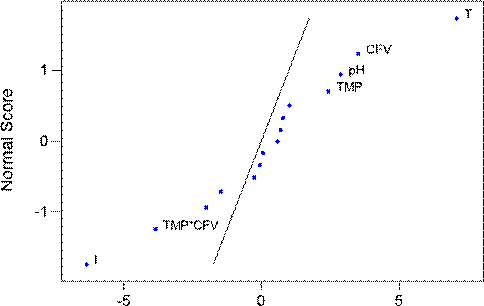
After 30 minutes filtration, the flux was close to the steady state. At this point in time, the results showed that all the five parameters (pH, TMP, CFV, T and I), together with TMP*CFV interaction effect significantly affected flux (shown in Fig.4). However, temperature and ionic strength effects still dominated the value of the flux.
It can be concluded that temperature is a very important parameter to control during filtration of particulate mixtures. It has a major effect on filtration flux at all stages of the experiments. However, as the filtration progresses from start-up through to steady state operation, other factors, such as ionic strength, begin to have an impact on performance. By the time steady state operation is achieved, all parameters investigated were making a significant contribution to the operating flux.
Standardized Effect
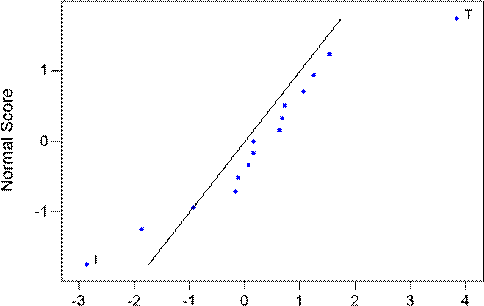
Fig. 3. Normal probability plot of effect of each factors on mid-run flux
Standardized Effect
Fig. 4. Normal probability plot of effect of each factors on steady state flux
Thanks to Institute of Environmental Science and Engineering (IESE), Singapore for providing a scholarship to support some of the research.
[1] R.W. Field, D. Wu, J.A. Howell, and B.B. Gupta, Critical flux concept for microfiltration fouling. Journal of Membrane Science, 1995. 100(3): p. 259-272.
[2] J.A. Howell, Sub-critical flux operation of microfiltration. Journal of Membrane Science, 1995. 107(1-2): p. 165-171.
[3] D. Wu, J.A. Howell, and R.W. Field, Critical flux measurement for model colloids.
Journal of Membrane Science, 1999. 152(1): p. 89-98.
[4] W. S. Winston Ho and K.K.S. (editors), Membrane Handbook. 1992, New York: Van Nostrand Reinhold.
[5] M. Mulder, Basic Principles of Membrane Technology. 2nd Ed. ed. 2000, Dordrecht: Kluwer Academic Publishers.
[6] M. Cheryan, Fouling and cleaning, in Ultrafiltration and Microfiltration Handbook. 1998, Technomic Publishing Company: Lancaster. p. 237-291.
[7] D.-J. Chang, F.-C. Hsu, and S.-J. Hwang, Steady-state permeate flux of cross-flow microfiltration. Journal of Membrane Science, 1995. 98(1-2): p. 97-106.
[8] Y.K. Benkahla, A. Ould-Dris, M.Y. Jaffrin, and D. Si-Hassen, Cake growth mechanism in cross-flow microfiltration of mineral suspensions. Journal of Membrane Science, 1995. 98(1-2): p. 107-117.
[9] Y. Zhao, J. Zhong, H. Li, N. Xu, and J. Shi, Fouling and regeneration of ceramic microfiltration membranes in processing acid wastewater containing fine TiO2 particles.
Journal of Membrane Science, 2002. 208(1-2): p. 331-341.
[10] H.K. Vyas, R.J. Bennett, and A.D. Marshall, Influence of operating conditions on membrane fouling in crossflow microfiltration of particulate suspensions. International Dairy Journal, 2000. 10(7): p. 477-487.
[11] K.-J. Hwang, Y.-H. Yu, and W.-M. Lu, Cross-flow microfiltration of submicron microbial suspension. Journal of Membrane Science, 2001. 194(2): p. 229-243.
[12] Q. Gan, J.A. Howell, R.W. Field, R. England, M.R. Bird, C.L. O'Shaughnessy, and M.T. MeKechinie, Beer clarification by microfiltration -- product quality control and fractionation of particles and macromolecules. Journal of Membrane Science, 2001. 194(2): p. 185-196.
[13] P. Aimar, M. Meireles, P. Bacchin, and V. Sanchez, Fouling and Concentration Polarisation in Ultrafiltration and Microfiltration, in Membrane Processes in Separation and Purification, J.G. Crespo, Editor. 1994, Kluwer Academic: Dordrecht. p. 27-57.
[14] W. Richard Bowen and A.O. Sharif, Hydrodynamic and colloidal interactions effects on the rejection of a particle larger than a pore in microfiltration and ultrafiltration membranes. Chemical Engineering Science, 1998. 53(5): p. 879-890.
[15] R.S. Faibish, M. Elimelech, and Y. Cohen, Effect of Interparticle Electrostatic Double Layer Interactions on Permeate Flux Decline in Crossflow Membrane Filtration of Colloidal Suspensions: An Experimental Investigation. Journal of Colloid and Interface Science, 1998. 204(1): p. 77-86.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.