For colloidal particles, particle stability and interactions are influenced by both solution ionic strength and pH. These effects are reflected in the filtration behaviour. The distance between each particle in the cake layer is shorter at high ionic strength because the repulsive forces between particles are reduced. As a result, the cake formed from a high ionic strength solution is more compact and has higher resistance [13]. Therefore, the permeate flux drops faster and reaches the steady-state flux faster at high ionic strength than at low ionic strength.
In this paper, a series of experiments focusing on the effects of combined feed properties (pH & ionic strength) and operating conditions (T, CFV, TMP) on permeate flux was carried out.
For the experiments, mixtures of silica (SiO2) and alumina (Al2O3) particles were prepared. The particles were kindly provided by Nissan Chemicals Co. (Japan). The primary particles were mono-dispersed, with a 20 nm diameter. All solutions and mixtures were made up using MilliQ water (> 18 MΩ/cm).
The particle size distributions of the mixtures were measured using a Malvern MastersizerE (Malvern Instruments, UK). The Malvern MastersizerE is a static small-angle laser light scattering (SALLS) instrument, which could measure particles from 0.5 µm to 600 µm using three different lenses. The 45 mm lens was used in this study. Because the particulate mixtures were very fragile, a syringe was used to inject the samples instead of the normal pumping circulation provided by the equipment.
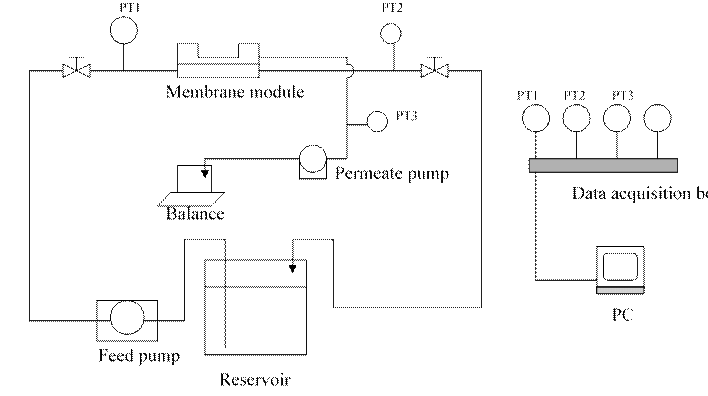
0.2 µm Millipore GVWP membranes were used in the filtration experiments. A crossflow micro-filtration rig shown in Fig.1 was used.
 Balance
Balance
Fig. 1. Cross-flow micro-filtration experiment set-up
Filtration mixture was prepared by combining silica and alumina sols, then adjusting the pH of the mixture to the desired value. The mixture was placed in a water bath and kept there for 30 minutes, after which the filtration experiment was commenced.
3.1 Characterization of particulate mixtures:
Silica (SiO2) particles are negatively charged and alumina (Al2O3) particles are positively charged. Due to the interaction between particles, the mixtures of silica and alumina form different structures under different solution conditions. When the pH of the mixture was low (~ 4), the mixtures were highly flocculated and formed larger and denser flocs (average particle size of 2.5±0.5 µm), which meant the particles were compacted together. When the mixture pH was high (~ 8), the mixture could not flocculate to form dense flocs (average particle size of 0.5±0.2 µm). The mixtures were well-dispersed sols. The particle size of this latter type of mixture was much smaller than the former.
3.2 Effects of Operating Conditions on Flux:
On completion of the filtration experiments, the effects of each factor on the flux were analysed using analysis of variance and normal probability plot techniques. Fig.2 shows the normal probability plot of the effects of the various parameters on the flux of the system shortly after the commencement of the experiments. The analysis showed that T and the pH/ionic strength interaction were the two factors that had the most significant impact on the starting flux of the particulate mixture. This could be due to the electro-static double layer interactions and hydrodynamic interaction between the colloidal particles. This result agrees with other results in the literature [14, 15] that flux strongly depends on ionic strength and pH due to the particle interaction. In addition, in this study, the pH and ionic strength could also affect particle size and size distribution in the mixture, which would also have an impact on filtration behaviour.
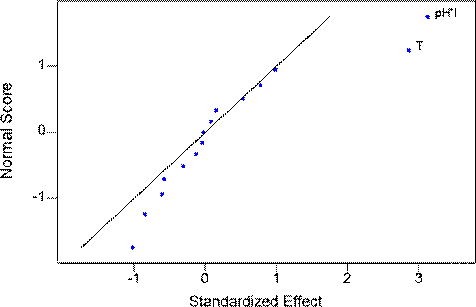
Fig. 2. Normal probability plot of effect of each factors on start-up flux
During the filtration process, the significant factors affecting the filtration behaviour changed. As shown in Fig.3, temperature and ionic strength became the significant factors that controlled the flux at the mid-point in the experiments, before steady-state flux was achieved. The interaction between pH and ionic strength was less important at this part.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.