Characterization The products were characterized by X-ray diffraction (XRD), using a D/max-γA diffractometer with Cu Kα radiation operated at 40 kV and 100 mA. Data were collected in the 2θ ranges from 10 °C to 70 °C (1= 0.15418 nm). The scanning electron microscope (SEM, Hitachi S-450, Japan) was used to observe the grain morphology and particle size. Infrared radiation (IR) results of the samples were determined by Perkin Elmer Spectrum one FTIR.
Electrode preparation and electrochemical performance test The cathode was prepared by mixing 80 wt% BaFeO4 (or BaFeO4&additives or TiO2-coated) with 15 wt% acetylene black and 5 wt% polyvinylidene fluoride, using N-methyl-2pyrrolidine as solvent. The additives are KMnO4, SrTiO3, and K2S2O8, respectively. After the electrode surface was treated by TiO2 sol, the electrode was dried under vacuum for 12 h. The mixed slurry was besmeared onto a foam nickel patch. The anode was commercial zinc and the electrolyte was 14 mol/L KOH. The mass of active materials was obtained by chemical method.
![]()
All the galvanostatic discharge tests were performed by using LandCT2001A Test System with current density of 0.5 mA/cm2 in the range of 0.8–2.0 V at room temperature.
Results and discussion
XRD analysis
The XRD pattern is shown in Fig. 2. All diffraction lines could be indexed as the orthorhombic system with a space group Td, which is consistent with the literatures reports [13, 14].
IR analysis
The IR spectrum of BaFeO4 is illustrated in Fig. 3, in which
−1 the peaks at 868, 814 and 779 cm are assigned to FeO4 tetrahedron stretching vibration peak, from which it can be seen that there is no triple degenerate at the fundamental frequency band but three detached peaks due to departure of
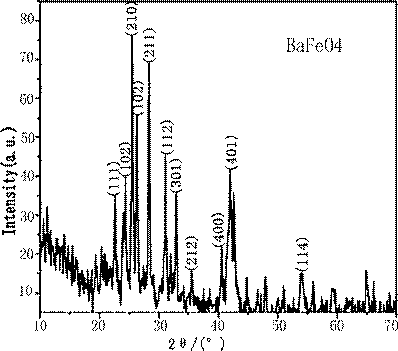
Fig. 2 XRD pattern of BaFeO4
|
87 Fig. 3 IR spectrum of BaFeO4 |
BaFeO4 to Td that agrees with what is reported [13, 14]. The
−1 peaks at 3,463 and 1,629 cm are assigned to stretching vibration absorption peak and bending vibration absorption
−1 peak of H2O. The peak at 1,384 cm is attributed to C-O stretching vibration absorption peak, this may due to the absorption of H2O and CO2 in air when tested.
SEM analysis
Figure 4 shows SEM image of BaFeO4. As can be seen from Fig. 4, the sample exhibits spherical morphology with average particle size of 50~100 nm.
Electrochemical performance
Figure 5 shows the discharge curves within the potential range 0.8–2.0 V at the current density of 0.5 mA/cm2. The discharge curve of BaFeO4 is shown in Fig. 5. The curve a is the discharging curve of BaFeO4 and the curves b, c, and d are the discharging curves of BaFeO4 with additive K2S2O8 of 15%, 10%, and 5%, respectively. From a comparison of a, b, c, and d in Fig. 5, some features were worthy to be mentioned. Firstly, the discharge capacity and the discharge platform of d are increased by 40 mAh/g and 0.3 V, respectively. Secondly, the discharge platform of c
descend and the smoothness is lower than a. Thirdly, the discharge capacity of b is improved unconspicuously and the platform also descend. The above results indicate that the electrochemical performance has been improved with adding K2S2O8. When 5% K2S2O8 is added into BaFeO4, both the discharge capacity and the discharge platform exhibit the best.
Figure 6 shows the effect of different additives on discharge performance of BaFeO4 electrode. The samples contain 5% of additives except for curve a is pure BaFeO4. As can be
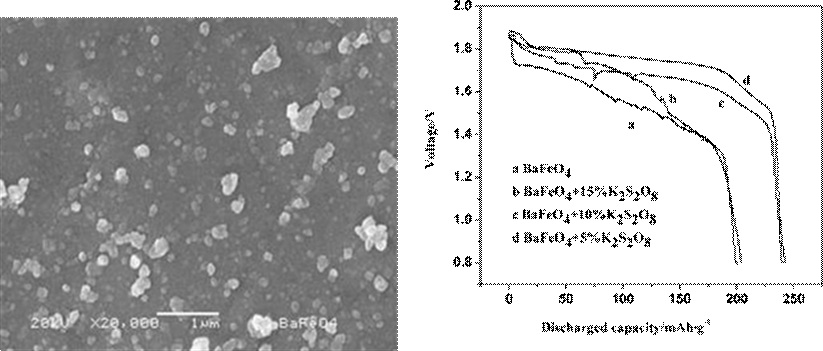
Fig. 5 Different contents of K2S2O8 effect on electrochemical
Fig. 4 SEM micrograph of BaFeO4 performance of BaFeO4 electrode
![]()
88 Ionics (2009) 15:85–89
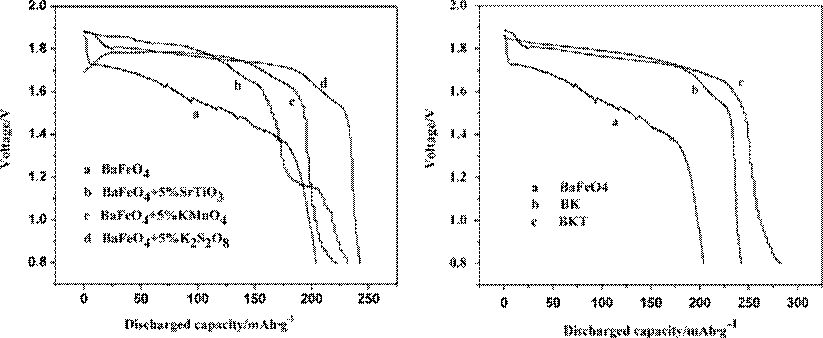
Fig. 6. Different additives effect on electrochemical performance of BaFeO4 electrode
seen from Fig. 6, the discharge capacity is the highest and the value is 240 mAh/g after adding 5% K2S2O8 (curve d) under the same condition. When KMnO4 and SrTiO3 are added, the discharge capacity is 220 mAh/g and 230 mAh/g, respectively. From the above results, the BaFeO4 with 5% K2S2O8 provides a flattest discharge profile and highest overall energy capacity.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.