
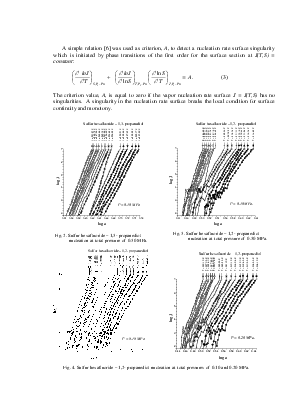
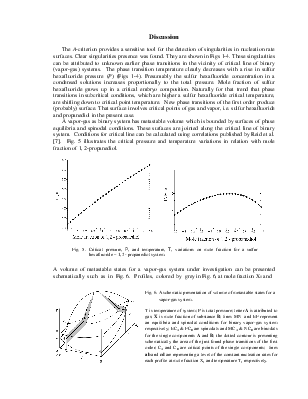
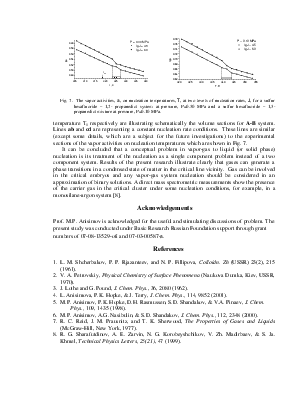
Geometry of Metastable State Volume for Vapor-Gas System and Experimental Data on Nucleation Rates in Critical Line Vicinity
Institute of Chemical Kinetics and Combustion, SB RAS, 3 Institutskaya Str., 630090 Novosibirsk, Russia
Abstract. The empirical results on nucleation of a vapor-gas systems show that in the vicinity of the critical line the phase transitions of the first order take place in a condensed matter. These phase transitions produce the nucleation rate surface singularities. The singularity examples for nucleation rate surfaces in a vicinity of critical line for several sulfur hexafluoride –propanediol systems are presented. A metastable state volume is designed semiempirically over the phase equilibria diagram for vapor-gas systems. Geometry of metastable state volume is studied qualitatively.
Introduction
Studies of vapor nucleation are of significant interest as this is a fundamentally important problem of the first-order phase formation kinetics description. The current technical level of research for the study of aerosol formation is of fairly high quality, but there is no theory that is suitable for quantitative prediction of the experimental vapor nucleation rates. In the common case, the theory of phase transitions cannot predict the phase transition pressure and temperature for nowadays. Theory involves a number of assumptions to describe small clusters. Further, when the size dependencies of the surface tension and density of nuclei were taken into account [1, 2] and the inherent degrees of freedom were used to calculate the statistical sum for a nascent cluster [3], agreement between theoretical and experimental results get worse.
Method and Results
A Laminar Flow Diffusion Chamber [4] is used for nucleation rate measurements of vapor-gas systems. Semiempirical design over diagram of phase equilibria is used for metastable volume construction [5].
|
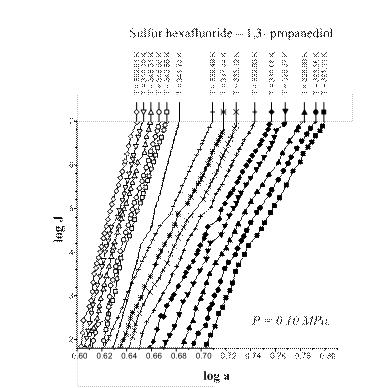
|
A simple relation [6] was used as criterion, A, to detect a nucleation rate surface singularity which is initiated by phase transitions of the first order for the surface section at J(T,S) = constant:
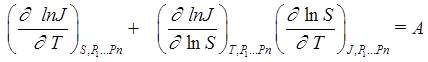 . (3)
. (3)
The criterion value, A, is equal to zero if the vapor nucleation rate surface J = J(T,S) has no singularities. A singularity in the nucleation rate surface breaks the local condition for surface continuity and monotony.

|
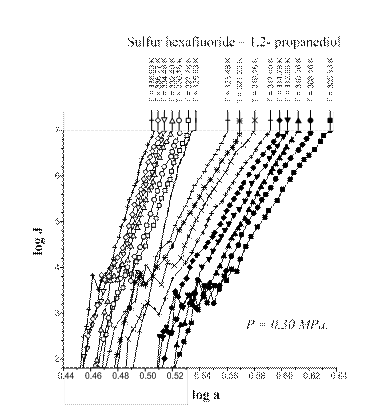
Fig. 2. Sulfur hexafluoride – 1,3- propanediol
nucleation at total pressure of 0.30 MPa.
|
Discussion
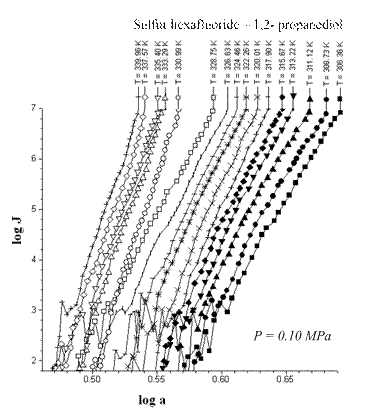
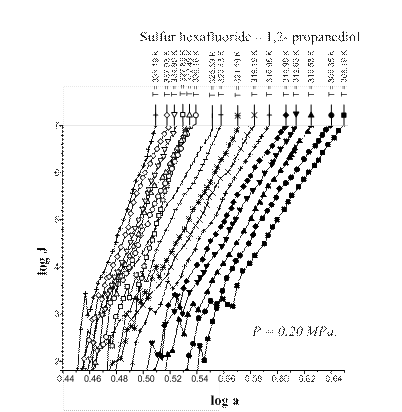
The A-criterion provides a sensitive tool for the detection of singularities in nucleation rate surfaces. Clear singularities presence was found. They are shown in Figs 1-4. These singularities can be attributed to unknown earlier phase transitions in the vicinity of critical line of binary (vapor-gas) systems. The phase transition temperature clearly decreases with a rise in sulfur hexafluoride pressure (P) (Figs 1-4). Presumably the sulfur hexafluoride concentration in a condensed solutions increases proportionally to the total pressure. Mole fraction of sulfur hexafluoride grows up in a critical embryo composition. Naturally for that trend that phase transitions in subcritical conditions, which are higher a sulfur hexafluoride critical temperature, are shifting down to critical point temperature. New phase transitions of the first order produce (probably) surface. That surface involves critical points of gas and vapor, i.e. sulfur hexafluoride and propanediol in the present case.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.