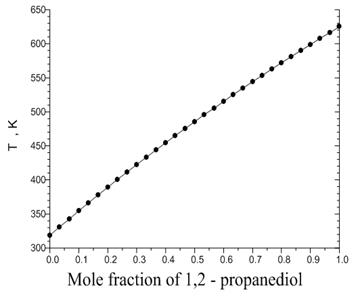
A vapor-gas as binary system has metastable volume which is bounded by surfaces of phase equilibria and spinodal conditions. These surfaces are jointed along the critical line of binary system. Conditions for critical line can be calculated using correlations published by Reid et al. [7]. Fig. 5 illustrates the critical pressure and temperature variations in relation with mole fraction of 1, 2-propanediol.
|
|
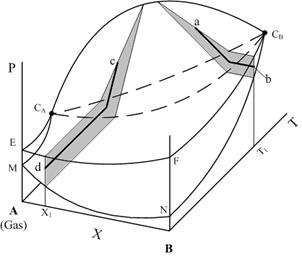
A volume of metastable states for a vapor-gas system under investigation can be presented schematically such as in Fig. 6. Profiles, colored by grey in Fig. 6, at mole fraction X1 and
|
|
|
|
|
Fig. 7. The vapor activities, a, on nucleation temperatures, T, at two levels of nucleation rates, J, for a sulfur hexafluoride – 1,2- propanediol system at pressure, P=0.30 MPa and a sulfur hexafluoride – 1,3- propanediol mixture at pressure, P=0.10 MPa.
temperature T1 respectively are illustrating schematically the volume sections for A-B system. Lines ab and cd are representing a constant nucleation rate conditions. These lines are similar (except some details, which are a subject for the future investigations) to the experimental sections of the vapor activities on nucleation temperatures which are shown in Fig. 7.
It can be concluded that a conceptual problem in vapor-gas to liquid (or solid phase) nucleation is its treatment of the nucleation as a single component problem instead of a two component system. Results of the present research illustrate clearly that gases can generate a phase transitions in a condensed state of matter in the critical line vicinity. Gas can be involved in the critical embryos and any vapor-gas system nucleation should be considered in an approximation of binary solutions. A direct mass spectrometric measurements show the presence of the carrier gas in the critical cluster under some nucleation conditions, for example, in a monosilane-argon system [8].
Acknowledgements
Prof. M.P. Anisimov is acknowledged for the useful and stimulating discussions of problem. The present study was conducted under Basic Research Russian Foundation support through grant numbers of 07-08-13529-ofi and 07-03-00587-а.
References
1. L. M. Shcherbakov, P. P. Rjazanteev, and N. P. Fillipova, Colloidn. Zh (USSR) 23(2), 215 (1961).
2. V. A. Petrovskiy, Physical Chemistry of Surface Phenomena (Naukova Dumka, Kiev, USSR, 1970).
3. J. Lothe and G. Pound, J. Chem. Phys., 36, 2080 (1962).
4. L. Anisimova, P.K. Hopke, & J. Terry, J. Chem. Phys., 114, 9852 (2001).
5. M.P. Anisimov, P.K. Hopke, D.H. Rasmussen, S.D. Shandakov, & V.A. Pinaev, J. Chem. Phys., 109, 1435 (1998).
6. M.P. Anisimov, A.G. Nasibulin, & S.D. Shandakov, J. Chem. Phys., 112, 2348 (2000).
7. R. C. Reid, J. M. Prausnitz, and T. K. Sherwood, The Properties of Gases and Liquids (McGraw-Hill, New York, 1977).
8. R. G. Sharafutdinov, A. E. Zarvin, N. G. Korobeyshchikov, V. Zh. Madirbaev, & S. Ja. Khmel, Technical Physics Letters, 25(21), 47 (1999).
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.