Практика 2
Провести расчеты трех реакторов.
Результаты оформить в виде отчета.
В отчете привести исходные данные, технологическую схему, вводимые параметры, полученные результаты и графики (если требуется)
1.Kinetic Reactor
Sodium acetate is prepared in a continuous reactor from the reaction of sodium hydroxide and ethyl acetate.
![]()
Sodium hydroxide and ethyl acetate are fed to the reactor at a concentration of 0.10 mol/L, a temperature of 30 °C and a pressure of 101325 Pa. The rate constant is 0.138 liter/mole*s. The volume of the reactor is 1L and the total feed flowrate is 0.086892 mol/s. Simulate the kinetic reactor and determined the outlet composition at steady state.
2. Stoichiometric Reactor
Problem Statement:
Reaction:
Decomposition of di-t-butyl peroxide (DTBP) to acetone and ethane
![]()
Reaction kinetics:
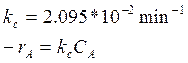
Reaction conditions:
Temperature 154.6 oC
Pressure 491.8 mmHg
Feed and feed conditions:
100 kmol/h of (DTBP) at 110 oC and 760 mmHg
Information for steady state:
Perform a stoichiometric reactor simulation to determine the heat of reaction and the steady-state mole rates of components in the product stream.
3.Plug Flow Reactor with Multiple Reactions
THE PROBLEM STATEMENT
Allyl chloride is to be produced in a 12-ft. long 2-in ID tube operating at isothermal PFR. The feed is a 4:1 molar ratio of propylene to chlorine and enters at a feed rate of 0.85 lbmole/hr and 2 atm of pressure and temperature of 1000 Rankine. The reactor pressure is assumed to be constant.
![]() ……………………Reaction1
……………………Reaction1
![]() …………………………Reaction2
…………………………Reaction2
The rate constants have units of lbmoles/(hr-ft3-atm2) and are:
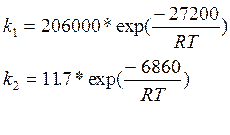
where T is in degrees Rankine and R is in Btu/(lbmole ºR). The rate expressions are
![]()
![]()
Calculate
1) The exiting stream concentrations from the reactor.
2) Plot the change in concentration of the reactants and products along the volume of the reactor.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.