(1) The curve for b1 = 0 corresponds to a case already examined and merely reproduces Fig. 10.3:
. For Ф > 10 we have h = 3/Ф.
. For Ф < 1 we have h» 1.
(2) For b1 <0 (endothermic reactions), the efficiency is always less than that corresponding to b1= 0.
(3) For b1 > 0 (exothermic reactions), the efficiency may be less than, equal to or greater than 1.
In a relatively restricted region, for low Ф and high b1, the curve h= f(Ф) can give three solutions for the same Ф. The physical significance of this occurrence is that, for the same external temperature Ts and the same surface concentration C1s (and hence the same b1and b2), two different theoretical states of stable operation of the particle may exist:
(a) A hot state, characterized by a high temperature gradient and an average operating temperature substantially higher than Ts, and also by a pronounced concentration gradient with an overall high reaction rate and hence very high efficiency, in the sense that we have defined.
(b) A cold state, characterized by gentle temperature and concentration gradients and efficiency generally barely higher than 1.
The third numerical solution, corresponding to intermediate efficiency, has no physical reality. We therefore felt it preferable to represent this portion of the region as shown in Fig. 10.6b, which marks this area in which two possible stable states arc observed for the particle.
The state assumed by the particle in this zone essentially depends on its previous thermal history. If the particle has been used with a slow approach from below to the operating temperature corresponding to an external temperature Ts, the stable state is the cold state. If, on the contrary, the particle has previously been raised to high temperature and it only gradually approaches its state of equilibrium in operation, it will evolve towards the hot state.
It may happen that unforeseen circumstances, operating irregularities, or runaway or extinguished reactions may cause a grain or granular mass to transfer from the hot state to the cold state, or vice versa, if this mass is in the zone of multiple stable states. This mechanism is detrimental to the operation of the reactor, and must be avoided if possible by altering the Thiele modulus, through the choice of the operating temperature, the control of heat exchange, or by any other means adapted to the circumstances.
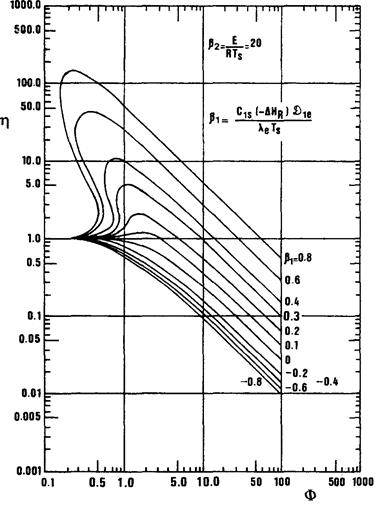 |
Fig. 10.6 a. Variation in efficiency h with Ф, b1 and b2 [3]. (The curve b1 = 0 repeats Fig. 10.3.)
 |
Fig. 10.6b. Efficiency h versus Ф for b2> 20 and b1 > 0.5. The possible stable states of a particle of porous catalyst.
10.4. SIMILARITY BETWEEN THE HATTA NUMBER AND THE THIELE MODULUS
To make a complete comparison of the two transfer mechanisms, in other words transfer from fluid phase I to a reaction fluid phase II, and transfer from a fluid phase I to a porous solid reaction phase II, it is first necessary to refer to an identical system of coordinates. Recalling that the fluid/fluid case was dealt with in Chapter 7 by reference to a plane interface, we must also treat the problem of intraparticulate diffusion with a plane fluid/solid interface. In this case (see note below) we can show that, for a first-order reaction, the efficiency h is expressed by:
(10.25)
where Ф' is a Thiele modulus defined by
(10.26)
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.