MODULUS AND EFFICIENCY [ 3]
The concepts of the Thiele modulus and efficiency can be extended to the following cases:
(a) Non-spherical grains.
(b) Reactions with an order different from 1.
(c) Non-isochoric reactions.
(d) Non-isothermal reactions.
10.3.1. Geometric shapes other than the sphere
For particles consisting of long cylinders or long plates, calculations similar to those presented for spheres can be carried out. In actual fact, it appears preferable to employ the concept of equivalent diameter, and to use a Thiele modulus defined as:
(10.16)
10.3.2. Reactions with an order different from 1
In the same way as in defining a modified Hatta number for fluid-fluid reactions with an order different from 1 for the transferred reactant, a Thiele modulus is defined and generalized to nth-order reactions with respect to reactant A1:
(10.17)
In the case of more complex kinetic expressions, although the identical calculations are sometimes possible analytically, it is necessary, depending on the type of kinetic expression:
(a) Either to treat the kinetics concerned as an nth -order reaction and to use Eq. (10.17).
(b) Or to integrate numerically the differential equation similar to Eq. (10.6) but containing the kinetic expression specific to the case concerned.
An elegant manner of dealing with the problem if the reaction order and rate constant are not simultaneously known, but if the reaction rate can be measured, consists in introducing the dimensionless Weisz number equal to Ф2h, as shown below.
If we recall that the observable local rate of disappearance of reactant A1 per unit volume of reactor (i.e. of catalyst bed) is equal to :
and that
we have
(10.18)
![]() Ф2h can be calculated from
the measured or estimated values of robs(A1), d1,
C1R, Д1e,. and ep.
Ф2h can be calculated from
the measured or estimated values of robs(A1), d1,
C1R, Д1e,. and ep.
Using the correlations for n = 0,1 and 2, it becomes possible to estimate hand hence to evaluate the degree of utilization of the catalyst for the reaction concerned (Fig. 10.4).
Figure 10.5 shows the concentration profile in terms of Ci/C^s according to the penetration depth z/R, for different orders of reaction, where
C1s = concentration of components A1 at the external surface of the particle,
C1s = C1R for a spherical particle.
10.3.3. Non-isochoric reactions
We have hitherto examined isochoric reactions such as:
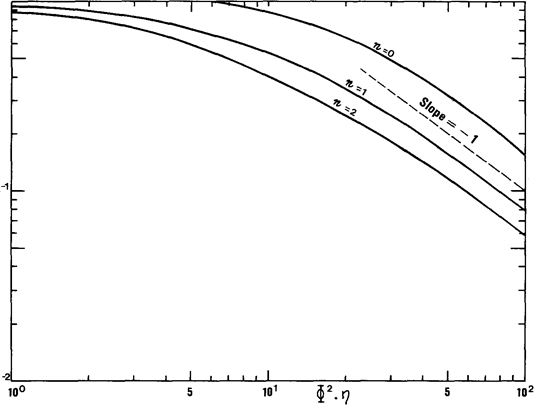
Fig. 10.4. Relationship between efficiency h and the function Ф2h
Isothermal, isochoric system, spherical geometry, order n.

Fig. 10.5. Concentration profiles in the grain, according to the value of Ф and order n.
In these conditions, for each molecule of A1 transformed in the grain, one mole of product is formed, which will travel in the opposite direction to A1 to leave the grain. This represents the case of equimolecular diffusion, to which the simplified form of Fick's law can be applied:
If v2 is different from — v1, the convection flux is superimposed on the diffusion flux and Fick's law is written:
with
= total concentration in mol/m3.
The writing of the mass balances then becomes complex, and it is impossible to find an analytical solution for C1z= f{z). As a rule:
(a) If v2 > (—v1) the efficiency decreases.
(b) If v2< (—v1), the efficiency increases.
Weekman and Gorring [4] introduced the volume change modulus :
(10.19)
where
C1s = concentration of reactant A1 at the external surface of the grain,
SCis = sum of the concentrations of all the compounds (including inerts) at the external surface of the grain,
and succeeded in calculating the ratio of efficiency hv (with change in volume) to that without change in volume h for different values of reaction order, Thiele modulus Ф and modulus w. Analyzing their conclusions, we can observe that hv /h remains between 0.7 and 1.3 for the usual values of:
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.