![]() The Nucleus, 46
(3) 2009: 205-211
The Nucleus, 46
(3) 2009: 205-211 ![]() The Nucleus
The Nucleus
A Quarterly Scientific Journal of Pakistan Atomic Energy Commission
NCLEAM, ISSN 0029-5698
ASSESSMENT AND IDENTIFICATION OF SOME NOVEL NOX REDUCING REAGENTS FOR SNCR PROCESS
A. MAHMOOD, *M. T. JAVED, N. IRFAN, A. HAMID and K. WAHEED1
Department of Chemical and Materials Engineering, P.O. PIEAS, Nilore, Islamabad, Pakistan 1
Department of Nuclear Engineering, PIEAS, Nilore, Islamabad, Pakistan
![]()
Nitrogen oxides (NOx) are one of the most hazardous air pollutants arising from the combustion processes. Because of the implementation of strict emission limits many NOx removal technologies have been developed. In the present work post combustion NOx removal technique that is Selective Non-Catalytic Reduction (SNCR) has been investigated in a pilot scale 150 kW combustion rig facility. Investigation has been performed using some novel NOx reducing reagents like urea, ammonium carbonate and mixture of their 50%-50% aqueous solution within the temperature range of 700 to 1200 oC, at 1.1% excess oxygen and background NOx level of 500 ppm. The effects of these reagents were determined in term of their temperature characteristics and molar ratio. Among the reducing reagents used urea solution gave the highest NOx removal efficiency (81%) and was attractive due to its superior high temperature (1000 to 1150 oC) performance, ammonium carbonate was more effective at lower temperature range (850 to 950 oC) though its efficiency (32%) was lower than urea, while 50-50% solution of urea and ammonium carbonate gave higher efficiency than ammonium carbonate but slightly lesser than urea within a wide temperature range (875 to 1125 oC). It was also observed that the NOx removal efficiency was increased with increasing the molar ratio.
Keywords: Nitrogen oxides (NOx), SNCR, Urea, Ammonium carbonate, Molar ratio
![]()
∗TP Corresponding author : m.t.javed@pieas.edu.pk PT
be developed to reduce the pollutants within the regulation limit from combustion processes. Numerous studies have been reported for costeffective NOx reduction from stationary combustion sources. These technologies include selective catalytic reduction (SCR) and selective noncatalytic reduction (SNCR). The SNCR process is a useful method for NOx reduction by injecting amines (–NH–) or cyanides (–CN–) containing selective reducing agents such as NH3, urea and cyanuric acid into the flue gases [1,2]. The SNCR process could reduce nitric oxides to nitrogen and water rapidly and effectively at rather higher (1073–1373 K) temperatures as compared to SCR [3]. It has been reported that injection of some additives together with the reducing agents in SNCR processes can lower and widen the optimum reaction temperature window for NOx reduction
[4, 5].
The experimental work was performed on the
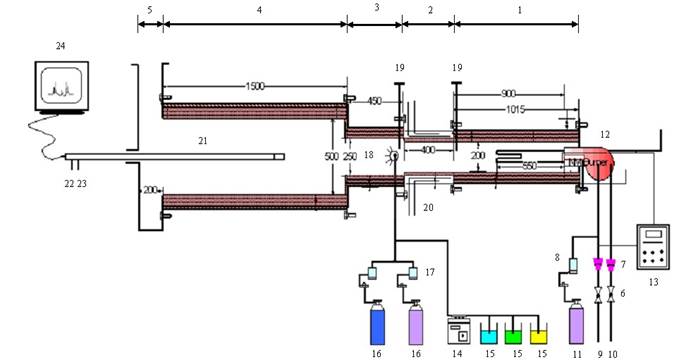
|
1. Combustion Section |
2. Temp. Control Section |
3. Injection Section |
4. Reaction Section |
|
5. Flue Gas Ehaust |
6. Control Valve |
7. Rota Meters |
8. Mass Flow Controller |
|
9. Methane Gas |
10. Air |
11. Ammonia (For B.G) |
12. Burner |
|
13. Control Panel |
14. Peristaltic Pump |
15. Liquid Reagents |
16. Carrier Gas (Air/N2) |
|
17. Pressure Guage |
18. Injector |
19. Thermocouples |
20. Cooling Coils |
|
21. Sampling Probe |
22. Water Inlet |
23. Water Outlet |
24. Flue Gas Analyzer |
Figure 1. Schematic representation of experimental setup.
“Combustion Rig” which we have developed in PIEAS and is a simulation of the modern fossil fuel fired power plant. The furnace was made of stainless steel and contains five sections as combustion section, temperature control section, injection and mixing section, reaction section and exhaust section (Figure 1). The other necessary equipments and the supply systems which were required to complete the setup include natural gas supply system, air supply system, ammonia supply system, thermocouples and suction pyrometer and flue gas analyzer. The detail of each section is given below:
Combustion section is the section where we are producing the flue gases according to our requirement. Combustion section is made of mild steel with the insulation of fire bricks, gas kits used to join combustion section with the other sections is made of asbestos.
Next to the combustion section is temperature control section
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.