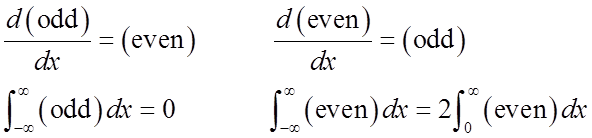
Just from symmetry:
![]()
![]()
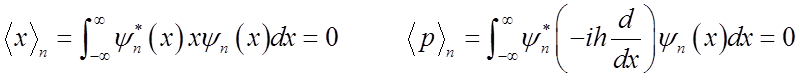
odd odd
Average displacement & average momentum = 0
IR spectroscopy Þ H.O. selection rules
 |
Intensity of vibrational absorption features
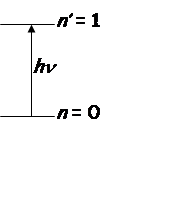 |
Vibrational transition
Intensity 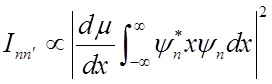
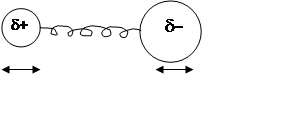
1) Dipole moment of molecule must change as molecule vibrates Þ
HCl can absorb IR radiation, but N2, O2, H2 cannot.
2) Only
transitions with ![]() allowed (selection
rule).
allowed (selection
rule).
(Prove for homework.)
|
|
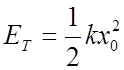
![]()
![]()
![]() oscillates between K and U.
oscillates between K and U.
Maximum displacement x0 occurs when
|
|
|
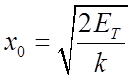 is the “classical turning point”
is the “classical turning point”
The classical oscillator with energy ET can never exceed this displacement, since if it did it would have more potential energy than the total energy.
QM H.O.
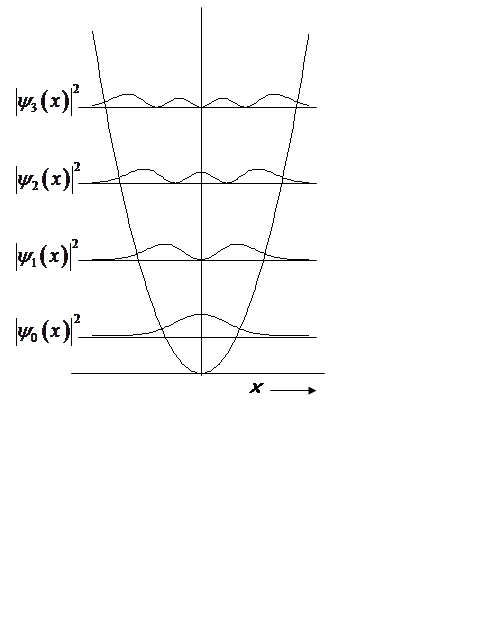
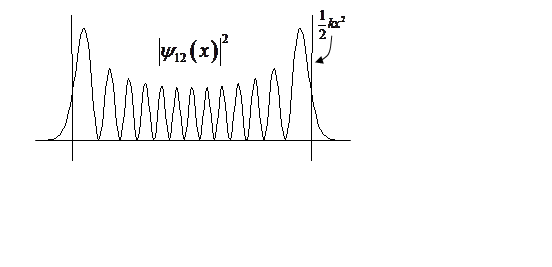
At high n, probability density begins to look classical, peaking at turning points.
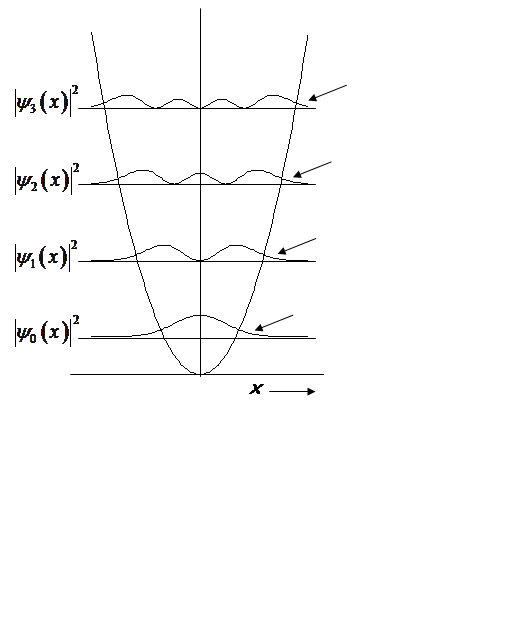
Non-zero probability at x > x0!
Prob. of (x > x0, x < -x0):
![]()
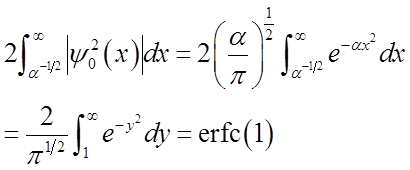 “Complementary error function”
“Complementary error function”
tabulated or calculated numerically
Prob. of (x > x0, x < -x0) = erfc(1) = 0.16
Significant probability!
The oscillator is “tunneling” into the classically forbidden region. This is a purely QM phenomenon!
Tunneling is a general feature of QM systems, especially those with very low mass like e- and H.
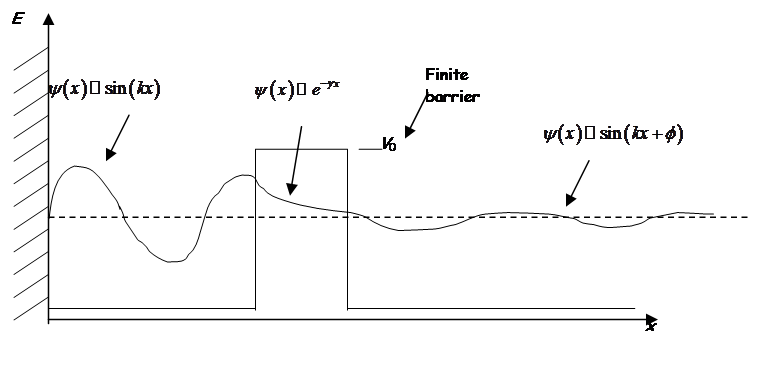
Even though the energy is less than the barrier height, the wavefunction is nonzero within the barrier! So a particle on the left may escape or “tunnel” into the right hand side.
Inside barrier: 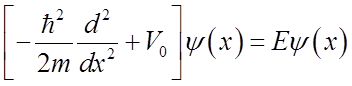
or 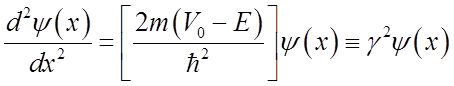
Solutions are of the form 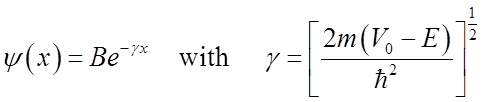
Note ![]()
If barrier is not too much higher then the energy and if the mass is light, then tunneling is significant.
Important for protons (e.g. H-bond fluctuations, tautomerization)
Important for electrons (e.g. scanning tunneling microscopy)
Nonstationary states of the QM H.O.
System may be in a state other than an eigenstate, e.g.
![]() with
with ![]() (normalization), e.g.
(normalization), e.g. 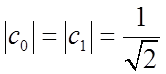
Full time-dependent eigenstates can be written as
![]()
where

System is then time-dependent:
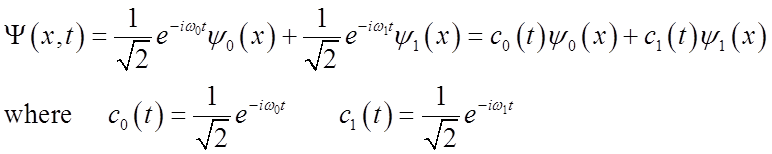
What is probability density?
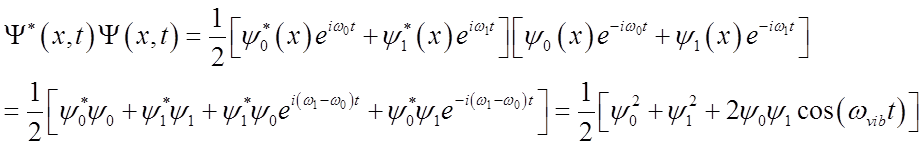
Probability density oscillates at the vibrational frequency!
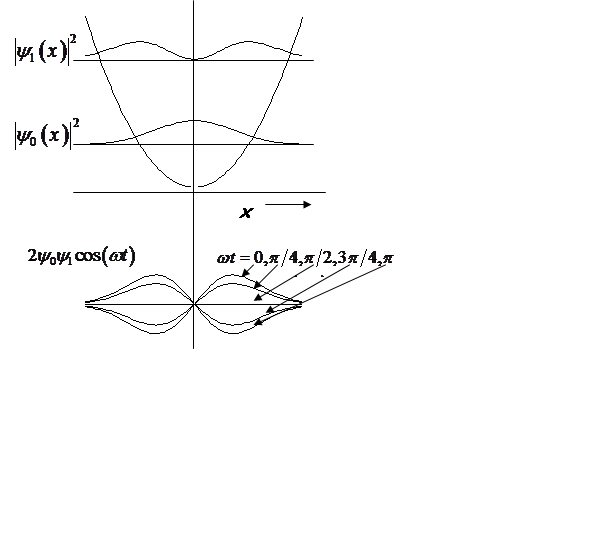 |
|||
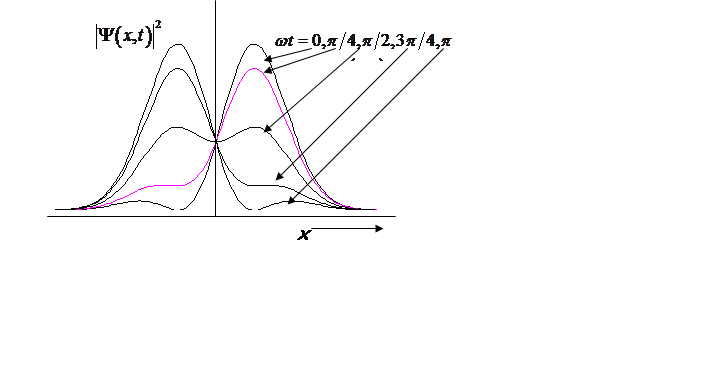 |
|||
What happens to the expectation value <x>?
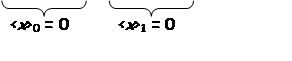
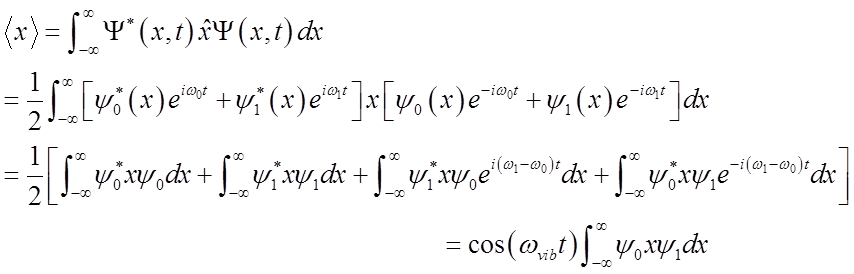
<x>(t) oscillates at the vibrational frequency, like the classical H.O.!
Vibrational
amplitude is 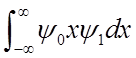
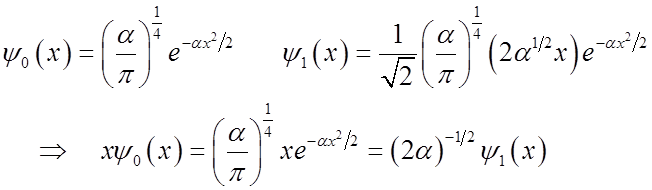
 \
\ 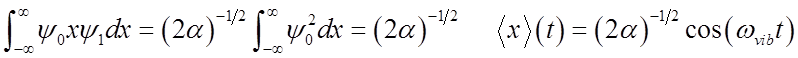
Relations among Hermite polynomials
Recall H.O. wavefunctions
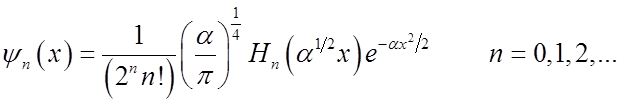
![]() Normalization Gaussian
Normalization Gaussian
Hermite polynomial
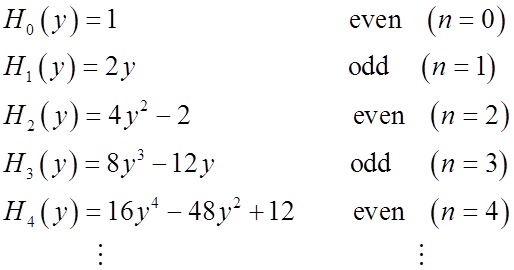
Generating formula for all the Hn:
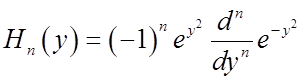
A useful derivative formula is:
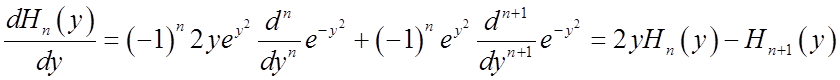
Another useful relation among the Hn’s is the recursion formula:
![]()
Substituting ![]() above gives
above gives
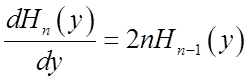
Use these relations to solve for momentum <p>(t)
|
|
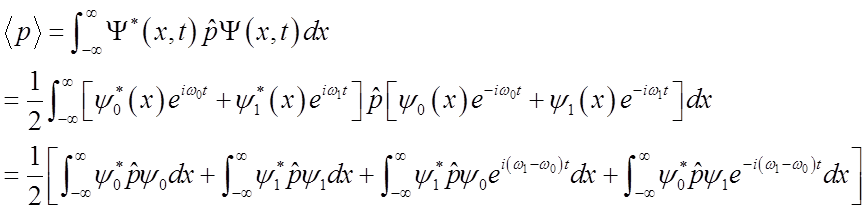
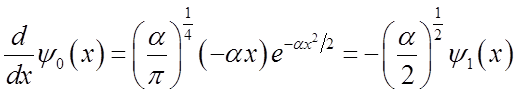
\ 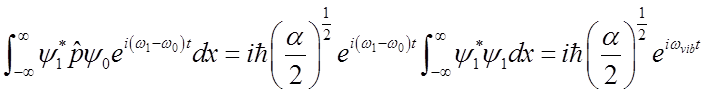
To solve
integral 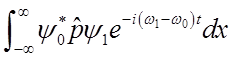 use relations among Hn’s
use relations among Hn’s
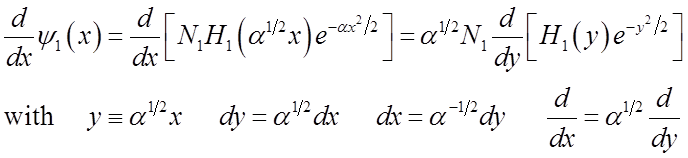
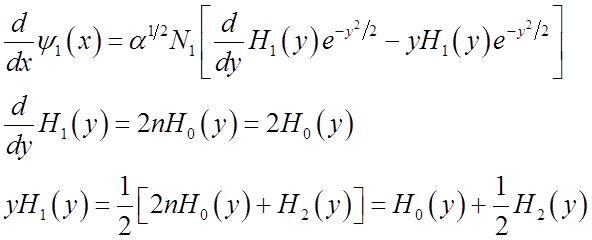
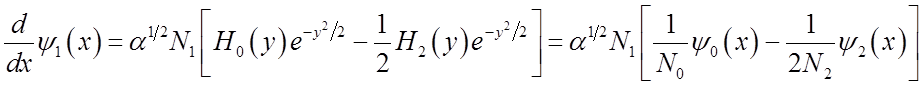
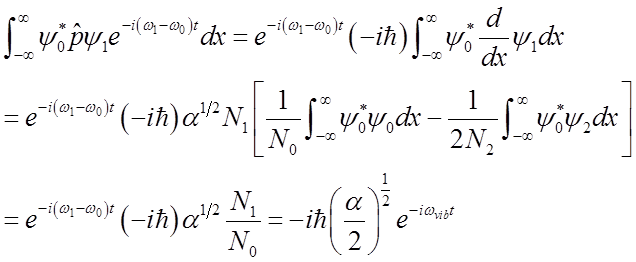
Finally
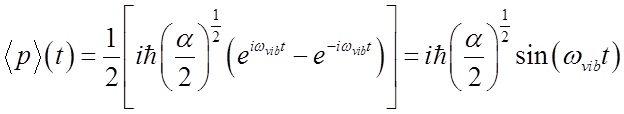
Average momentum also oscillates at the vibrational frequency.
Уважаемый посетитель!
Чтобы распечатать файл, скачайте его (в формате Word).
Ссылка на скачивание - внизу страницы.